IB MYP 4-5 Physics- Nuclear stability and isotopes- Study Notes - New Syllabus
IB MYP 4-5 Physics-Nuclear stability and isotopes- Study Notes
Key Concepts
- Nuclear stability and isotopes
Nuclear Stability and Isotopes
Nuclear Stability and Isotopes
Nuclear Stability
The nucleus of an atom is made up of protons (positively charged) and neutrons (neutral).
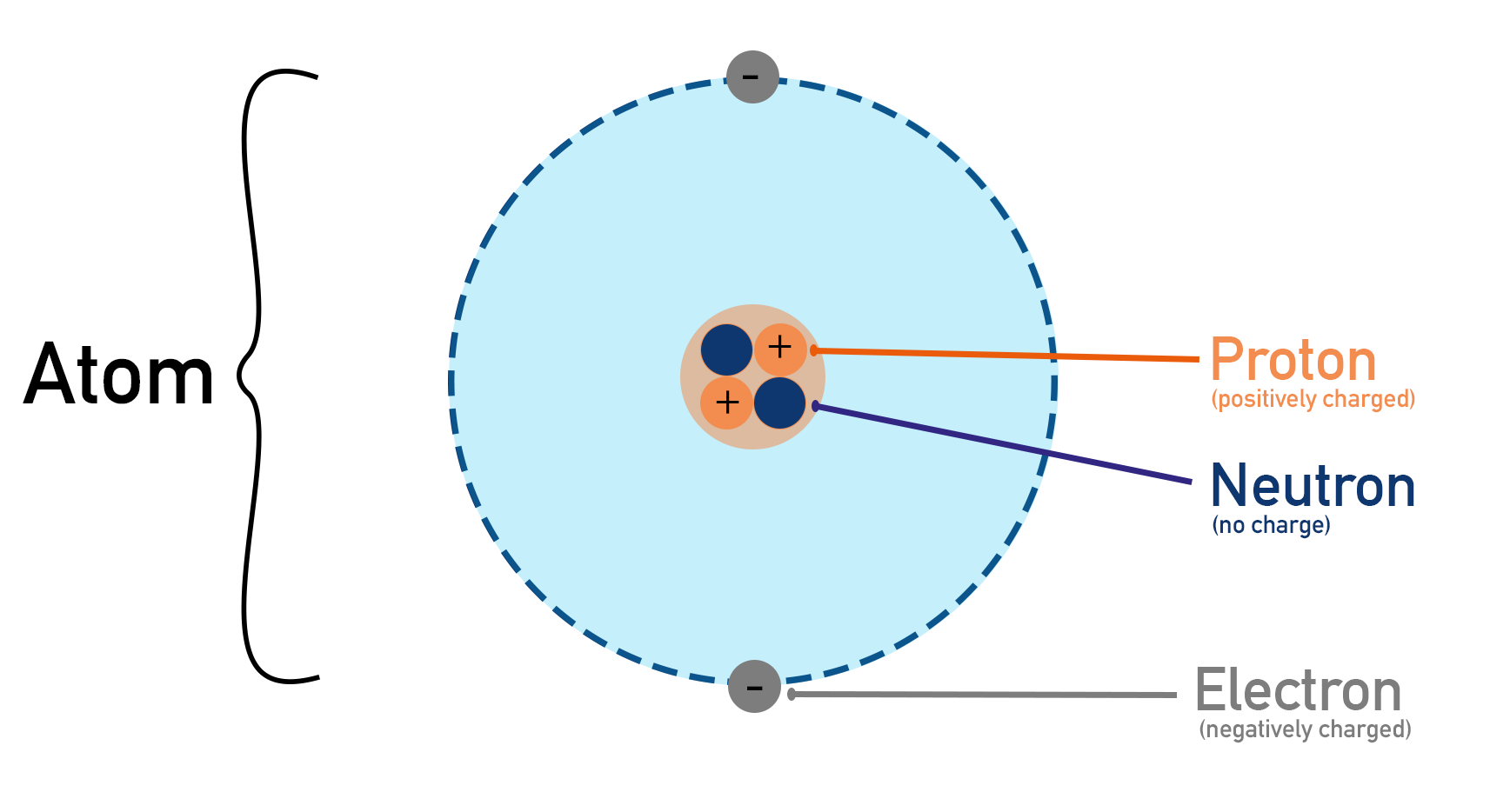
- Protons repel each other due to electrostatic (Coulomb) repulsion, but they are held together by the strong nuclear force, which acts only at very short distances.
- A nucleus is stable if the number of neutrons and protons are balanced in such a way that the strong nuclear force is strong enough to overcome the repulsion between protons.
- For lighter elements (like carbon, oxygen), stability usually requires roughly equal numbers of protons and neutrons.
- For heavier elements (like uranium), more neutrons than protons are required for stability to “dilute” the repulsion between many protons.
- If a nucleus is unstable, it undergoes radioactive decay to achieve stability.
Isotopes
Isotopes are atoms of the same element that have the same number of protons (same atomic number, Z) but different numbers of neutrons (different mass numbers, A).
- Since they have the same number of protons, isotopes belong to the same element and have the same chemical properties.
- However, isotopes differ in their mass, nuclear stability, and sometimes in their radioactivity.
Examples:

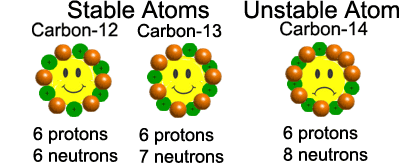
\(\ ^{12}C \) (6 protons, 6 neutrons) → Stable
\(\ ^{14}C \) (6 protons, 8 neutrons) → Radioactive
Stable vs. Unstable Isotopes
- Stable isotopes: Do not undergo radioactive decay. Example: \(\ ^{12}C\), \(\ ^{16}O\).
- Unstable isotopes (radioisotopes): Have too many or too few neutrons compared to protons. They decay by emitting radiation (alpha, beta, gamma). Example: \(\ ^{14}C\), \(\ ^{238}U\).
Notes:
- Nuclear stability depends on the balance between protons and neutrons.
- Isotopes have the same atomic number but different mass numbers.
- Stable isotopes are non-radioactive, while unstable isotopes undergo decay to become more stable.
Example:
An atom has 6 protons and 7 neutrons. Identify the isotope and state whether it is stable or unstable.
▶️ Answer/Explanation
Step 1: Number of protons = 6 → The element is Carbon (C).
Step 2: Mass number \( A = \text{protons + neutrons} = 6 + 7 = 13 \).
Isotope = \( ^{13}C \).
Step 3: \( ^{13}C \) is stable because it has a neutron-to-proton ratio close to 1:1.
Final Answer: \(\boxed{^{13}C \text{ (Stable isotope)}}\)
Example:
Uranium-238 has 92 protons and 146 neutrons. Explain why this isotope is radioactive.
▶️ Answer/Explanation
Step 1: Proton number = 92 → Element is Uranium (U).
Step 2: Neutron-to-proton ratio = \( \dfrac{146}{92} \approx 1.59 \).
Step 3: For heavy elements, too many neutrons are required to balance proton repulsion. In this case, even with 146 neutrons, the nucleus is still unstable.
Step 4: Uranium-238 undergoes alpha decay to reduce its size and move towards stability.
Final Answer: \(\boxed{^{238}U \text{ is unstable and radioactive due to excess neutrons.}}\)
Example:
Hydrogen has three isotopes: \( ^{1}H \) (1 proton, 0 neutrons), \( ^{2}H \) (1 proton, 1 neutron), and \( ^{3}H \) (1 proton, 2 neutrons). Which are stable and which is unstable?
▶️ Answer/Explanation
Step 1: \( ^{1}H \) → Proton only. Stable (ordinary hydrogen).
Step 2: \( ^{2}H \) → 1 proton + 1 neutron. Stable (called deuterium).
Step 3: \( ^{3}H \) → 1 proton + 2 neutrons. Too many neutrons, unstable (called tritium).
Final Answer: \(\boxed{^{1}H \text{ and } ^{2}H \text{ are stable; } ^{3}H \text{ is unstable (radioactive).}}\)
