IB MYP 4-5 Physics- Radioactivity and nuclear radiation- Study Notes - New Syllabus
IB MYP 4-5 Physics-Radioactivity and nuclear radiation- Study Notes
Key Concepts
- Radioactivity and nuclear radiation
Radioactivity and Nuclear Radiation
Radioactivity and Nuclear Radiation
Radioactivity
Radioactivity is the spontaneous emission of radiation from unstable atomic nuclei.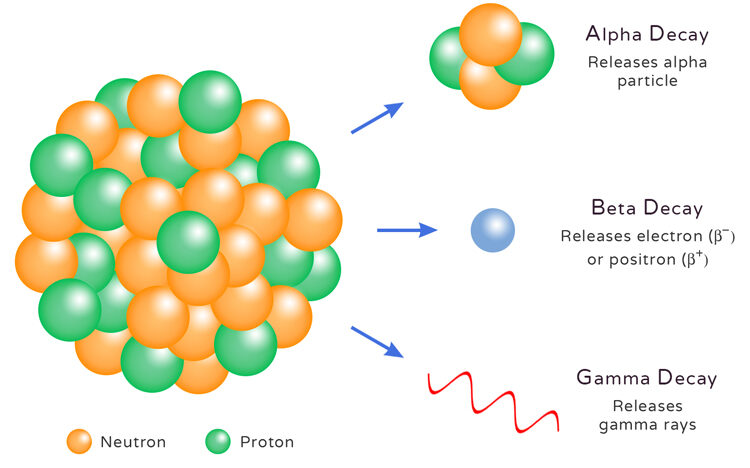
- Unstable nuclei try to become more stable by giving out particles or energy.
- This process does not require any external energy input—it happens naturally.
Causes of Radioactivity
- In very large atoms (like uranium), the repulsive force between protons is too strong for the nuclear force to hold them together.
- In atoms with too many neutrons or too few neutrons, the balance of forces is unstable.
- As a result, the nucleus emits radiation to reach stability.
Types of Nuclear Radiation

Alpha (α) radiation:
- Helium nucleus (2 protons + 2 neutrons).
- Highly ionizing but low penetration (stopped by paper or skin).
- Causes the nucleus to lose 2 protons and 2 neutrons.
Beta (β) radiation:
- A high-speed electron (β⁻) or positron (β⁺).
- Moderate penetration (stopped by a thin sheet of aluminum).
- In β⁻ decay: a neutron turns into a proton and an electron is emitted.
Gamma (γ) radiation:
- High-energy electromagnetic wave.
- Very penetrating (needs thick lead or concrete to stop).
- Does not change proton or neutron numbers—just releases extra energy.
Nuclear Equations
- Radioactive decays can be represented using nuclear equations.
- Example:
\( ^{238}_{92}U \;\;\rightarrow\;\; ^{234}_{90}Th \;+\; ^{4}_{2}He \) (alpha decay)
Properties of Nuclear Radiation

- Alpha: highly ionizing, least penetrating.
- Beta: medium ionization and penetration.
- Gamma: least ionizing but most penetrating.
Dangers of Nuclear Radiation
- Can damage living tissues and DNA, leading to cancer.
- Causes ionization in cells, which may kill or mutate them.
- Requires shielding and safety measures in laboratories and nuclear power plants.
Uses of Radioactivity
- Medical: cancer treatment (gamma rays), medical tracers (radioactive isotopes).
- Industrial: thickness measurement, leak detection.
- Energy: nuclear power generation (controlled fission).
- Archaeology: carbon dating (using carbon-14).
Example
Complete and balance the alpha-decay equation for Polonium-210:
\( \;^{210}_{84}\text{Po} \;\rightarrow\; \;^{4}_{2}\text{He} \;+\; \;^{\;\;?\;}_{\;?}\text{X} \)
▶️ Answer / Explanation
Step 1: Conserve mass number \(A\): \(210 = 4 + A_X \Rightarrow A_X = 210 – 4 = 206\).
Step 2: Conserve atomic number \(Z\): \(84 = 2 + Z_X \Rightarrow Z_X = 84 – 2 = 82\).
Step 3: Element with \(Z=82\) is Lead (Pb).
Balanced equation: \( \;^{210}_{84}\text{Po} \;\rightarrow\; \;^{4}_{2}\text{He} \;+\; \;^{206}_{82}\text{Pb} \).
Example
Carbon-14 undergoes beta− decay. Write the nuclear equation and identify the daughter nuclide.
▶️ Answer / Explanation
Key idea: In β− decay, a neutron → proton + electron (and an antineutrino). Mass number stays the same; atomic number increases by 1.
Equation: \( \;^{14}_{6}\text{C} \;\rightarrow\; \;^{14}_{7}\text{N} \;+\; \;^{0}_{-1}e \;+\; \bar{\nu}_e \).
Daughter nuclide: \( \;^{14}_{7}\text{N} \) (Nitrogen-14).
Example
A lab stores three sealed sources: α, β, and γ. For each, choose a suitable primary shield and a handling precaution. Briefly justify.
▶️ Answer / Explanation
Alpha (α): Shield — paper or thin plastic; Precaution — prevent ingestion/inhalation (use closed containers, fume hood). Reason: Very low penetration but highly ionizing inside the body.
Beta (β): Shield — few mm of aluminum or acrylic; Precaution — wear gloves, use tongs, avoid bremsstrahlung by not using very high-Z shields. Reason: Moderate penetration; interactions can create secondary X-rays in dense materials.
Gamma (γ): Shield — thick lead or concrete; Precaution — maximize distance and minimize time (tongs, remote handling), use dosimeter. Reason: Highly penetrating; follow time–distance–shielding principles.
