IB MYP 4-5 Physics- Relationship between gas pressure and volume- Study Notes - New Syllabus
IB MYP 4-5 Physics-Relationship between gas pressure and volume- Study Notes
Key Concepts
- Relationship between gas pressure and volume
Relationship between Gas Pressure and Volume
Relationship between Gas Pressure and Volume
The relationship between the pressure and volume of a fixed mass of gas at constant temperature is described by Boyle’s Law.

Boyle’s Law: For a fixed mass of gas at constant temperature, the pressure is inversely proportional to the volume.
Mathematically: \( P \propto \dfrac{1}{V} \) or \( P V = \text{constant} \)
- P = Pressure of the gas (Pa)
- V = Volume of the gas (m³)
- Temperature remains constant.
- If the volume is halved, the pressure is doubled (and vice versa).
Explanation:
- Gas particles are in constant random motion, colliding with the container walls.
- Reducing volume increases the frequency of collisions, increasing pressure.
- Increasing volume decreases collision frequency, lowering pressure.
Formula for calculations:
\( P_1 V_1 = P_2 V_2 \) (for constant temperature and fixed mass of gas)
Graphical Representation:
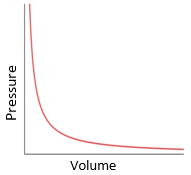

- Pressure vs Volume graph: A curve (hyperbola) showing inverse proportionality.
- Pressure vs 1/Volume graph: A straight line through the origin showing direct proportionality.
Example:
A gas at pressure \( 200 \, \text{kPa} \) has a volume of \( 0.04 \, \text{m}^3 \). If the volume is increased to \( 0.06 \, \text{m}^3 \) at constant temperature, find the new pressure.
▶️ Answer/Explanation
Using \( P_1 V_1 = P_2 V_2 \):
\( 200 \times 0.04 = P_2 \times 0.06 \)
\( 8 = 0.06 P_2 \)
\( P_2 = \dfrac{8}{0.06} = 133.33 \, \text{kPa} \)
Final Answer: \(\boxed{133.33 \, \text{kPa}}\)
Example:
If a gas-filled syringe is pushed in slowly without heating, what happens to the gas pressure and why?
▶️ Answer/Explanation
Pushing the syringe reduces volume → molecules collide more frequently with the walls → pressure increases according to Boyle’s Law.
