IB MYP 4-5 Physics- Simple kinetic molecular model of matter- Study Notes - New Syllabus
IB MYP 4-5 Physics- Simple kinetic molecular model of matter- Study Notes
Key Concepts
- Mappings
Simple Kinetic Molecular Model of Matter
Simple Kinetic Molecular Model of Matter
The kinetic molecular model of matter explains the physical properties and behaviour of solids, liquids, and gases in terms of the motion and arrangement of particles (atoms or molecules).
It forms the basis for understanding changes of state, gas laws, and thermal properties.
Key Assumptions of the Kinetic Molecular Model:
- All matter is made up of a large number of very small particles (atoms or molecules).
- These particles are in constant, random motion.
- Collisions between particles (and with container walls) are perfectly elastic — no net loss of kinetic energy.
- The average kinetic energy of particles is directly proportional to the absolute temperature \( T \) (in Kelvin).
- Intermolecular forces determine the arrangement and spacing of particles.
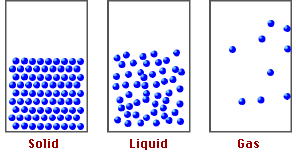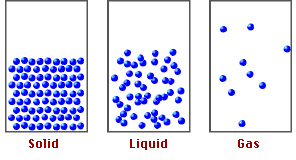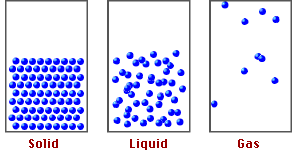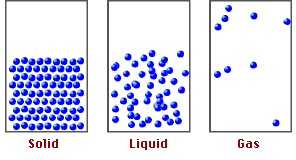
States of Matter and Particle Behavior:
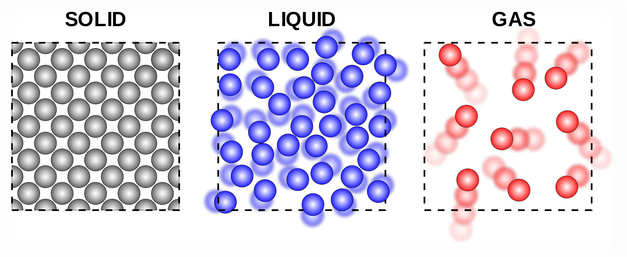
Solids:
- Particles are closely packed in a fixed, regular arrangement (lattice structure).
- Particles vibrate about fixed positions but do not move freely.
- Strong intermolecular forces keep particles in place.
- Solids have fixed shape and volume.
Liquids:
- Particles are close together but not in a fixed arrangement.
- Particles can slide past each other, allowing liquids to flow.
- Intermolecular forces are weaker than in solids but still significant.
- Liquids have fixed volume but no fixed shape.
Gases:
- Particles are far apart with negligible intermolecular forces.
- Particles move freely and rapidly in all directions.
- Collisions between particles and walls cause gas pressure.
- Gases have neither fixed shape nor fixed volume — they expand to fill their container.
Temperature and Kinetic Energy Relationship:

Average kinetic energy per particle: \( E_k = \dfrac{3}{2} k_B T \) (where \( k_B \) is Boltzmann’s constant).
An increase in temperature increases the average speed of the particles.
Changes of State in Kinetic Theory:
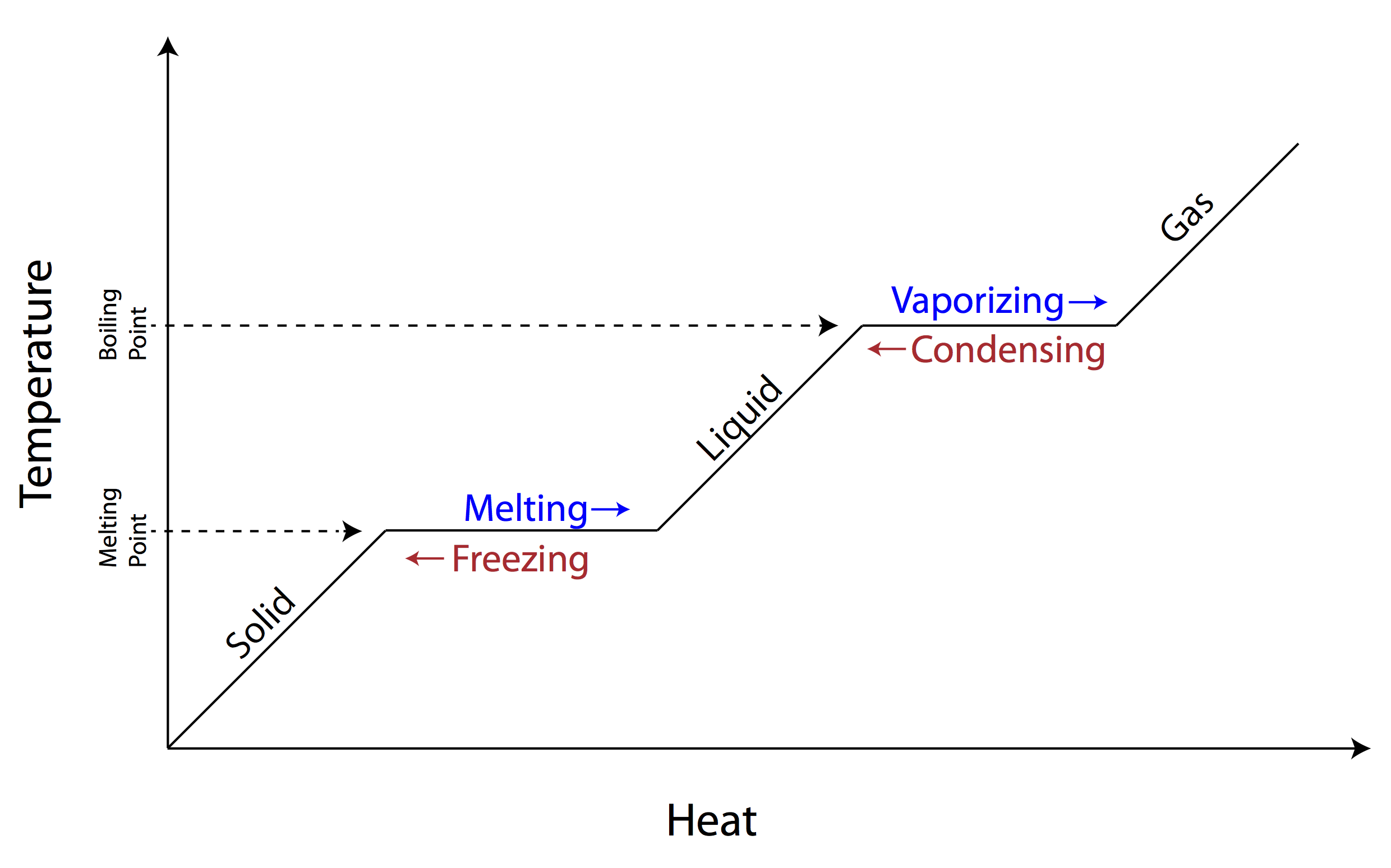
- Melting: Particles gain enough energy to break some intermolecular bonds and move freely.
- Freezing: Particles lose kinetic energy, bonds reform, and structure becomes fixed.
- Boiling/Evaporation: Particles gain enough kinetic energy to overcome all intermolecular forces and escape into the gas phase.
- Condensation: Gas particles lose energy and intermolecular forces pull them closer together into a liquid.
- Sublimation: Direct change between solid and gas without passing through liquid phase.
Gas Pressure in Kinetic Theory:

- Gas pressure is caused by collisions of gas particles with container walls.
- The greater the number and force of collisions, the higher the pressure.
- For a fixed amount of gas at constant temperature: \( pV = \text{constant} \) (Boyle’s Law).
Limitations of the Kinetic Molecular Model:
- Does not account for quantum effects at very low temperatures.
- Real gases deviate from ideal behavior at high pressures and low temperatures due to intermolecular forces.
Comparative Table of Particle Arrangement in Different States:
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Arrangement | Regular, fixed | Irregular, close | Random, far apart |
| Motion | Vibration only | Slide past each other | Move freely at high speed |
| Forces | Strong | Moderate | Negligible |
| Energy | Lowest | Moderate | Highest |
Example:
A sealed container of gas is heated from \( 27^\circ\text{C} \) to \( 127^\circ\text{C} \) while the volume remains constant. If the initial pressure is \( 100 \, \text{kPa} \), calculate the final pressure. Assume ideal gas behavior.
▶️ Answer/Explanation
Step 1: Convert temperatures to Kelvin:
\( T_1 = 27 + 273 = 300 \, \text{K} \)
\( T_2 = 127 + 273 = 400 \, \text{K} \)
Step 2: Use Gay-Lussac’s law \( \dfrac{P_1}{T_1} = \dfrac{P_2}{T_2} \):
\( \dfrac{100}{300} = \dfrac{P_2}{400} \)
Step 3: Solve for \( P_2 \):
\( P_2 = \dfrac{100 \times 400}{300} = 133.33 \, \text{kPa} \)
Final Answer: \( \boxed{133.33 \, \text{kPa}} \)
Example:
A fixed mass of gas occupies \( 0.5 \, \text{m}^3 \) at \( 200 \, \text{kPa} \). If the pressure is increased to \( 400 \, \text{kPa} \) at constant temperature, find the final volume.
▶️ Answer/Explanation
Step 1: Use Boyle’s law \( P_1 V_1 = P_2 V_2 \):
\( 200 \times 0.5 = 400 \times V_2 \)
Step 2: Solve for \( V_2 \):
\( V_2 = \dfrac{100}{400} = 0.25 \, \text{m}^3 \)
Final Answer: \( \boxed{0.25 \, \text{m}^3} \)
Example:
Why does a car tire’s pressure increase after a long drive on a hot day?
▶️ Answer/Explanation
When the car moves, friction between the tires and the road generates heat. This heat transfers to the air inside the tire, increasing the average kinetic energy of the gas molecules. According to the kinetic molecular model, higher temperature means faster-moving molecules, which collide with the walls more frequently and with greater force, thereby increasing the pressure.
