IB MYP 4-5 Physics- Specific heat capacity - Study Notes - New Syllabus
IB MYP 4-5 Physics-Specific heat capacity – Study Notes
Key Concepts
- Specific heat capacity
Specific Heat Capacity (SHC)
Specific Heat Capacity (SHC)
The Specific Heat Capacity of a substance is the amount of heat energy required to raise the temperature of 1 kilogram of the substance by 1°C (or 1 K).
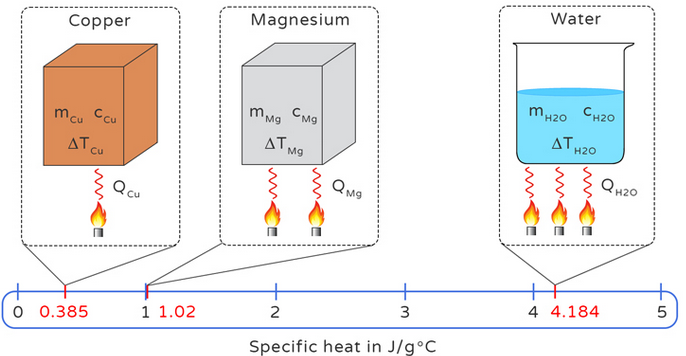

- Q = heat energy supplied or removed (Joules, J)
- m = mass of the substance (kilograms, kg)
- c = specific heat capacity (J·kg\(^{-1}\)·°C\(^{-1}\) or J·kg\(^{-1}\)·K\(^{-1}\))
- ΔT = change in temperature (°C or K)

Key Points:
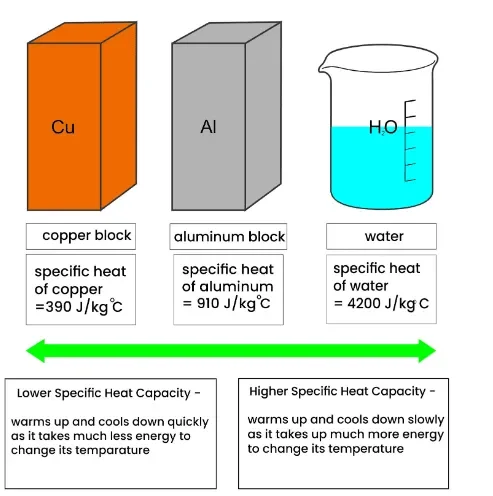
- The higher the SHC, the more energy is needed to raise the temperature of the substance.
- Water has a very high SHC (\( c \approx 4200\ \mathrm{J·kg^{-1}·°C^{-1}} \)), making it useful for cooling systems.
- Metals generally have low SHCs, meaning they heat up and cool down quickly.
Factors Affecting Specific Heat Capacity:
- Material type : different substances have different SHCs due to molecular structure.
- Temperature range : SHC can slightly change with temperature.
- Phase (solid, liquid, gas) : SHC varies in different states of matter.
Applications of Specific Heat Capacity:
- Cooking utensils: Metals with low SHC heat up quickly.
- Car radiators: Water absorbs a lot of heat without a large rise in temperature.
- Climate moderation: Oceans store heat and regulate climate.
Example:
A 2 kg block of aluminum (\(c = 900\ \mathrm{J·kg^{-1}·°C^{-1}}\)) is heated from 20°C to 50°C. Find the heat required.
▶️ Answer/Explanation
Using \( Q = mc\Delta T \)
\( Q = (2)(900)(50 – 20) \)
\( Q = 2 \times 900 \times 30 \)
\( Q = 54{,}000\ \mathrm{J} \)
\(\boxed{54\ \mathrm{kJ}}\)
Example:
How much heat is required to raise the temperature of 500 g of water from 25°C to 100°C?
▶️ Answer/Explanation
Mass \( m = 0.5\ \mathrm{kg} \), \( c = 4200\ \mathrm{J·kg^{-1}·°C^{-1}} \)
\( Q = mc\Delta T \)
\( Q = 0.5 \times 4200 \times (100 – 25) \)
\( Q = 0.5 \times 4200 \times 75 \)
\( Q = 157{,}500\ \mathrm{J} \)
\(\boxed{157.5\ \mathrm{kJ}}\)
Example:
Why does water take longer to heat than sand under the same sunlight?
▶️ Answer/Explanation
Water has a much higher SHC compared to sand, meaning it needs more heat energy to raise its temperature by the same amount. Sand, with a lower SHC, heats up and cools down quickly, while water resists temperature changes.
