IB MYP 4-5 Physics- Specific latent heat- Study Notes - New Syllabus
IB MYP 4-5 Physics-Specific latent heat- Study Notes
Key Concepts
- Specific latent heat
Specific Latent Heat (SLH)
Specific Latent Heat
The specific latent heat of a substance is the amount of heat energy required to change the state of 1 kg of the substance without any change in temperature.
\( L = \dfrac{Q}{m} \)
- \( L \) = specific latent heat (J/kg)
- \( Q \) = heat energy supplied or removed (J)
- \( m \) = mass of the substance (kg)
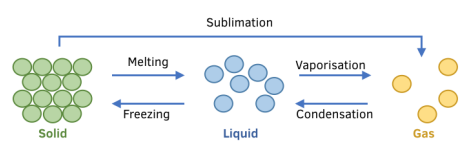
Types of Specific Latent Heat:

Specific Latent Heat of Fusion (\( L_f \))
Heat energy required to change 1 kg of a substance from solid to liquid (melting) or liquid to solid (freezing) without temperature change.
Specific Latent Heat of Vaporization (\( L_v \))
Heat energy required to change 1 kg of a substance from liquid to gas (boiling) or gas to liquid (condensation) without temperature change.
Important Points:
- During a phase change, the temperature of the substance remains constant even though heat is being transferred.
- This heat is used to break or form intermolecular bonds rather than increase kinetic energy of particles.
- Heating curves and cooling curves show flat regions where phase change occurs.
Formula for Heat Energy during Phase Change:
\( Q = m \times L \)
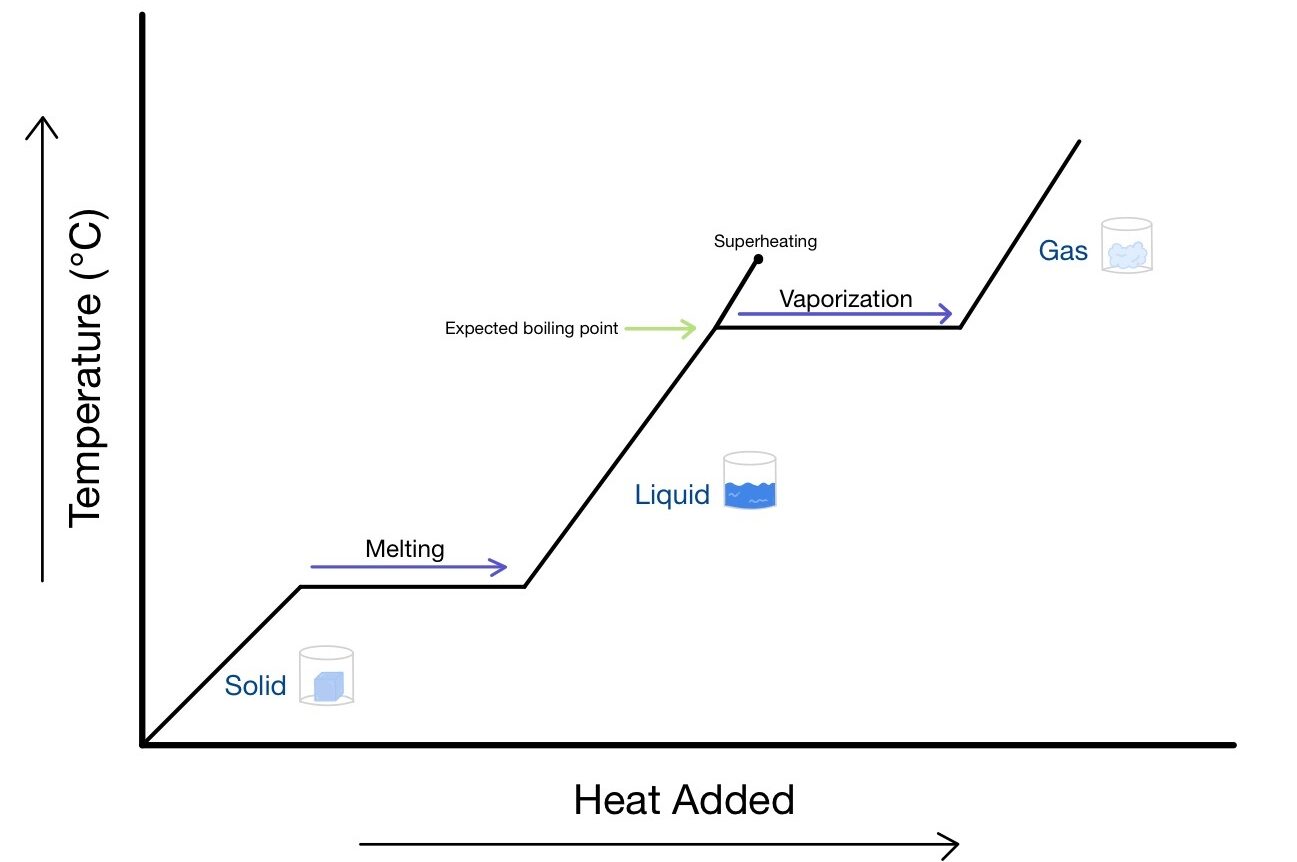
Heating Curve:
- Solid heats up → Temperature rises
- Melting → Temperature constant at melting point, latent heat of fusion absorbed
- Liquid heats up → Temperature rises
- Boiling → Temperature constant at boiling point, latent heat of vaporization absorbed
Everyday Applications:
- Ice packs: Use latent heat absorption to cool injuries without changing temperature.
- Steam burns: Steam has high latent heat of vaporization; releases large energy when condensing on skin, causing more severe burns than hot water.
- Climate regulation: Large water bodies absorb/release latent heat, moderating temperature changes in nearby regions.
Example:
Calculate the heat energy required to melt 0.5 kg of ice at 0°C. The specific latent heat of fusion of ice is \( 3.34 \times 10^5 \ \text{J/kg} \).
▶️ Answer/Explanation
\( Q = m \times L_f \)
\( Q = 0.5 \times 3.34 \times 10^5 = 1.67 \times 10^5 \ \text{J} \)
\(\boxed{1.67 \times 10^5 \ \text{J}}\)
Example:
How much energy is required to completely boil away 2 kg of water at 100°C? The specific latent heat of vaporization of water is \( 2.26 \times 10^6 \ \text{J/kg} \).
▶️ Answer/Explanation
\( Q = m \times L_v \)
\( Q = 2 \times 2.26 \times 10^6 = 4.52 \times 10^6 \ \text{J} \)
\(\boxed{4.52 \times 10^6 \ \text{J}}\)
Example:
Why does boiling water remain at 100°C until all the water turns into steam, even though heat is continuously supplied?
▶️ Answer/Explanation
During boiling, the heat energy supplied is used to break the intermolecular bonds between water molecules rather than increase their kinetic energy. Since temperature is a measure of average kinetic energy, it remains constant during the phase change.
