IB MYP 4-5 Physics- The gas laws- Study Notes - New Syllabus
IB MYP 4-5 Physics-The gas laws- Study Notes
Key Concepts
- The gas laws
The Gas Laws
The Gas Laws
The gas laws describe the relationship between pressure (\(P\)), volume (\(V\)), and temperature (\(T\)) of a fixed mass of gas. They are based on the Kinetic Molecular Theory, which assumes that gas particles move randomly, collide elastically, and exert pressure on container walls.
Key Assumptions of Ideal Gases:

- Gas particles are point masses with negligible volume compared to the container.
- Collisions between particles and walls are perfectly elastic.
- No intermolecular forces exist except during collisions.
- The average kinetic energy of gas particles is directly proportional to the absolute temperature (\(T\) in Kelvin).
1. Boyle’s Law
At constant temperature, the pressure of a fixed mass of gas is inversely proportional to its volume.
P \(\propto\) \(\dfrac{1}{V}\)
\(P_1 V_1 = P_2 V_2\)
- If volume decreases, pressure increases because particles collide more often with the walls.
2. Charles’s Law
At constant pressure, the volume of a fixed mass of gas is directly proportional to its absolute temperature.
V \(\propto T\) (in Kelvin)
\(\dfrac{V_1}{T_1} = \dfrac{V_2}{T_2}\)
This graph shows that at constant pressure, the volume of the given sample of the gas is directly proportional to the kelvin temperature.
- If temperature increases, volume increases because particles move faster and spread further apart.
3. Pressure Law (Gay-Lussac’s Law)
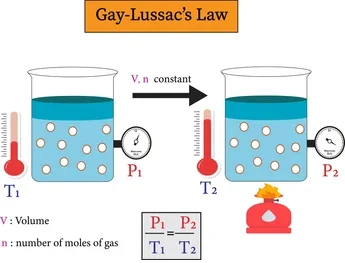
At constant volume, the pressure of a fixed mass of gas is directly proportional to its absolute temperature.
P \(\propto T\) (in Kelvin)
\(\dfrac{P_1}{T_1} = \dfrac{P_2}{T_2}\)
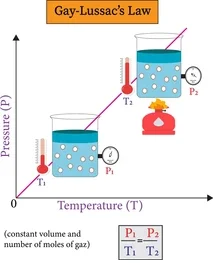
- If temperature increases, pressure increases because particles hit container walls more frequently and with greater force.
4. Combined Gas Law
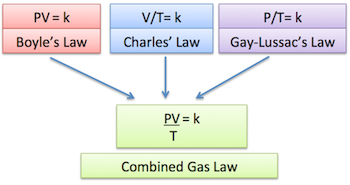
When none of the three variables (P, V, T) are constant, the relationships combine into:
\(\dfrac{P_1 V_1}{T_1} = \dfrac{P_2 V_2}{T_2}\)
5. Ideal Gas Equation

This general equation combines all gas relationships into one formula:
\(P V = n R T\)
- \(P\) = pressure (Pa)
- \(V\) = volume (m³)
- \(n\) = number of moles
- \(R\) = gas constant (\(8.314 \, \text{J mol}^{-1} \text{K}^{-1}\))
- \(T\) = temperature (K)
Everyday Applications of Gas Laws:
- Inflating a balloon (Boyle’s & Charles’s Law)
- Pressure changes in aerosol cans with temperature (Pressure Law)
- Hot-air balloons rising due to heated gas expansion (Charles’s Law)
Example:
A gas at 300 kPa has a volume of 2.0 L. If the volume changes to 3.5 L at constant temperature, find the new pressure.
▶️ Answer/Explanation
Using \(P_1 V_1 = P_2 V_2\):
\(300 \times 2.0 = P_2 \times 3.5\)
\(P_2 = \dfrac{600}{3.5} = 171.43 \, \text{kPa}\)
\(\boxed{171.43 \, \text{kPa}}\)
Example:
A gas has a volume of 1.5 L at 300 K. What will its volume be at 450 K if pressure is constant?
▶️ Answer/Explanation
Using \(\dfrac{V_1}{T_1} = \dfrac{V_2}{T_2}\):
\(\dfrac{1.5}{300} = \dfrac{V_2}{450}\)
\(V_2 = \dfrac{1.5 \times 450}{300} = 2.25 \, \text{L}\)
\(\boxed{2.25 \, \text{L}}\)
Example:
A gas has a pressure of 150 kPa at 280 K. What will its pressure be at 350 K if volume remains constant?
▶️ Answer/Explanation
Using \(\dfrac{P_1}{T_1} = \dfrac{P_2}{T_2}\):
\(\dfrac{150}{280} = \dfrac{P_2}{350}\)
\(P_2 = \dfrac{150 \times 350}{280} = 187.5 \, \text{kPa}\)
\(\boxed{187.5 \, \text{kPa}}\)
