IB MYP 4-5 Physics- Thermal processes- Study Notes - New Syllabus
IB MYP 4-5 Physics-Thermal processes- Study Notes
Key Concepts
- Thermal processes
Thermal Processes
Thermal Processes
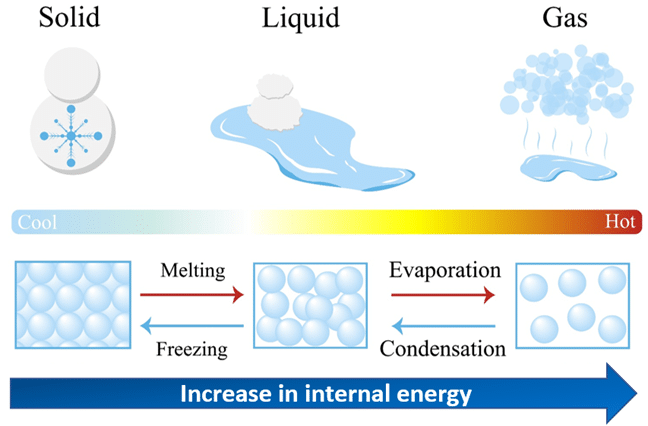
Thermal processes refer to the physical changes in matter that occur due to the transfer of thermal energy. These processes involve changes in temperature, state, or internal energy of a substance without focusing on the method of heat transfer.
Heating:
Heating increases the internal energy of a substance. The added energy may raise the temperature (increasing the kinetic energy of particles) or cause a change of state (affecting the potential energy between particles).
Cooling:
Cooling removes thermal energy from a substance, reducing the kinetic energy of particles. This can lower the temperature or lead to a change of state (e.g., gas condensing to liquid).
Melting (Fusion):

The process where a solid changes into a liquid at its melting point. Thermal energy is absorbed, increasing the potential energy of particles without changing their kinetic energy — hence temperature remains constant during melting.
Freezing (Solidification):

The reverse of melting, where a liquid changes into a solid at its freezing point. Energy is released to the surroundings as the potential energy of particles decreases, but temperature remains constant until solidification is complete.
Evaporation:

A process in which particles at the surface of a liquid gain enough energy to escape into the gas phase, occurring below the boiling point. It depends on temperature, surface area, humidity, and airflow.
Boiling:

The rapid vaporization of a liquid when it reaches its boiling point, occurring throughout the liquid. Thermal energy is absorbed to break intermolecular bonds, with temperature constant until the phase change is complete.
Condensation:

The process in which a gas changes into a liquid when it loses energy. Particle kinetic energy decreases, allowing intermolecular attractions to form, releasing latent heat.
Sublimation:
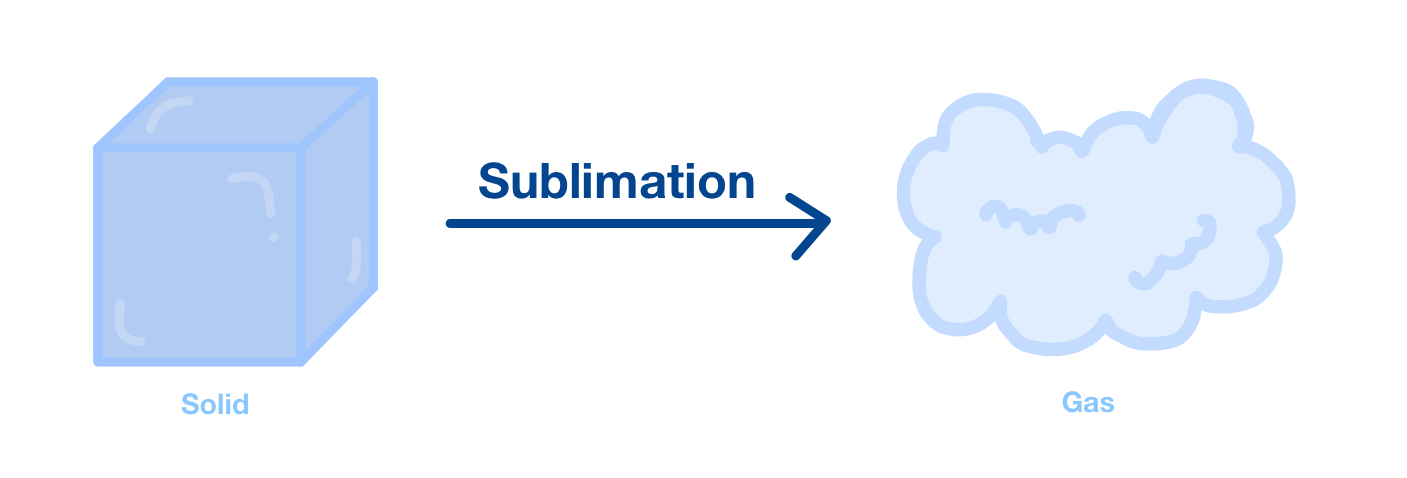
The change of state from solid to gas without passing through the liquid state. This requires a large amount of energy to overcome intermolecular forces (e.g., dry ice).
Deposition:
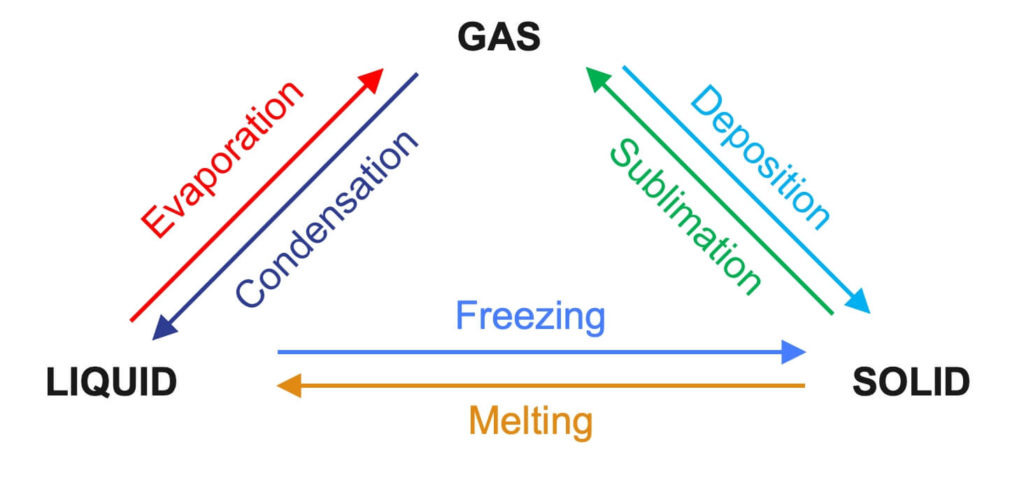
The change from gas directly to solid without becoming liquid first (e.g., frost formation). Energy is released to the surroundings.
Example:
A block of ice at \(0^\circ\text{C}\) is left on a table at room temperature (\(25^\circ\text{C}\)). Describe the sequence of thermal processes that will occur until the ice turns completely into water.
▶️ Answer/Explanation
Step 1: Initially, thermal energy from the surroundings is absorbed by the ice without raising its temperature. This energy is used to break intermolecular bonds — this is melting.
Step 2: During melting, temperature remains constant at \(0^\circ\text{C}\) until all ice turns into water.
Step 3: Once melting is complete, further heating increases the kinetic energy of the water molecules, raising the water’s temperature — this is heating.
Example:
On a cold morning, you notice frost forming on the leaves of plants. Identify the thermal process involved and explain it in terms of particle behavior.
▶️ Answer/Explanation
Step 1: The frost forms directly from water vapor in the air changing into ice without becoming liquid first — this is deposition.
Step 2: As water vapor particles lose kinetic energy, they slow down and settle into fixed positions, forming a solid structure.
Step 3: During deposition, latent heat is released to the surroundings, which is why the process often occurs on very cold surfaces.
Example:
When a kettle is boiling, bubbles form throughout the water, and steam is produced. Explain what thermal process is occurring and why the temperature remains constant.
▶️ Answer/Explanation
Step 1: The water is undergoing boiling, where liquid water changes into water vapor throughout the liquid, not just at the surface.
Step 2: Even though energy is continuously supplied, the temperature remains constant at the boiling point (\(100^\circ\text{C}\) at standard pressure) because all the energy is used to break intermolecular forces rather than increase kinetic energy.
Step 3: This absorbed energy is called the latent heat of vaporization.
