IB MYP 4-5 Physics- Thermal properties and Temperature- Study Notes - New Syllabus
IB MYP 4-5 Physics-Thermal properties and Temperature- Study Notes
Key Concepts
- Thermal properties and Temperature
Thermal Properties and Temperature
Thermal Properties and Temperature
Concept of Temperature
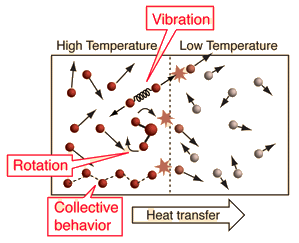
- Temperature is a measure of the average kinetic energy of particles in a substance.
- It indicates how hot or cold an object is, and determines the direction of heat transfer.
- Heat flows from higher temperature regions to lower temperature regions until thermal equilibrium is reached.
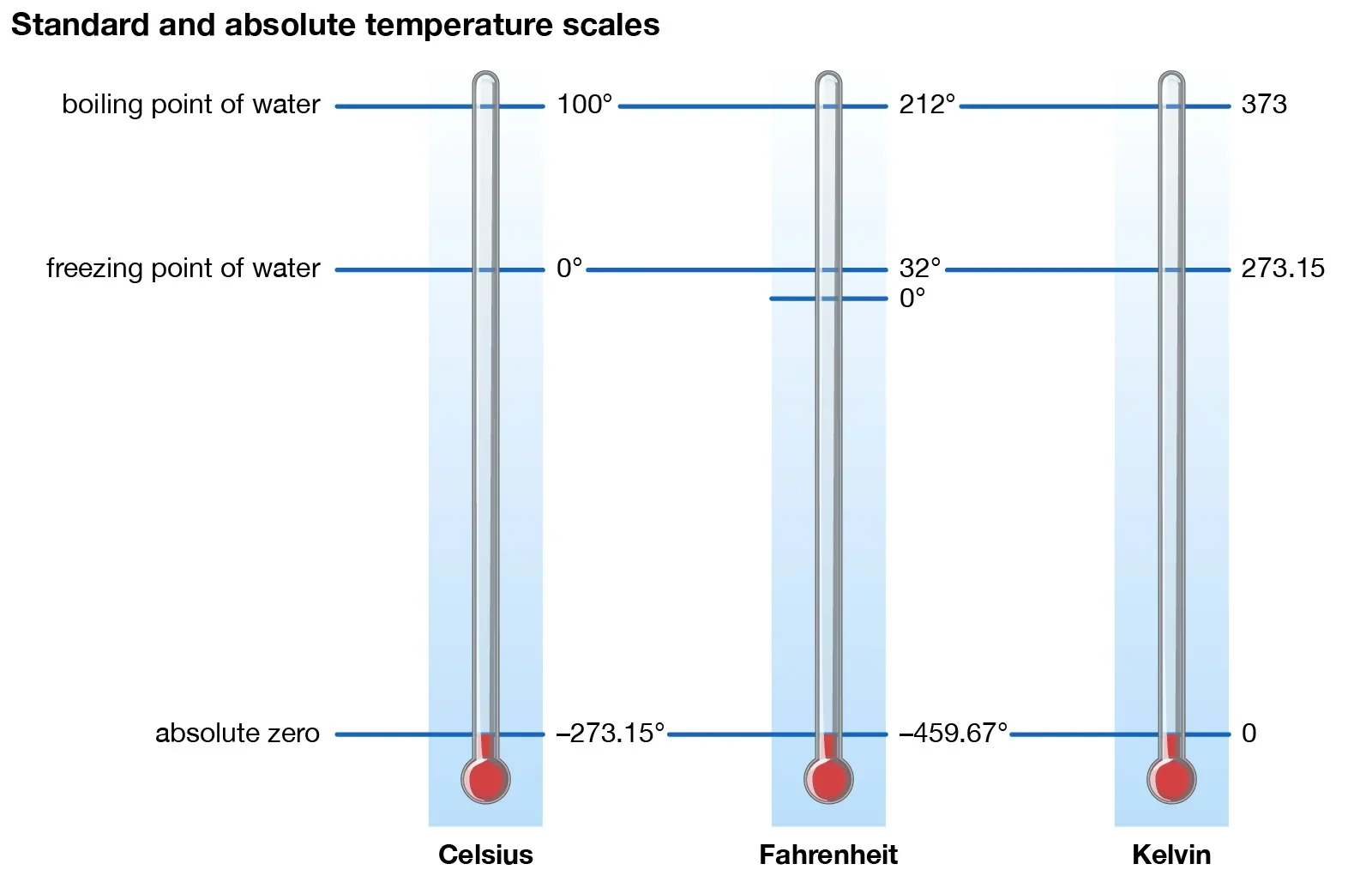
Measured in degrees Celsius (°C), Kelvin (K), or Fahrenheit (°F).
Relationship Between Heat, Temperature, and Internal Energy
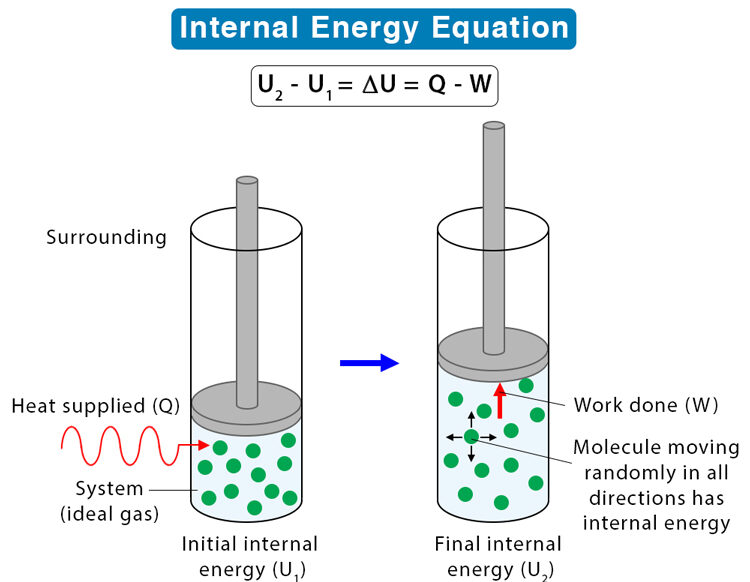
- Internal energy is the total kinetic and potential energy of particles in a substance.
- Heat is the energy transferred due to a temperature difference.
- Temperature is proportional to the average kinetic energy of particles, but internal energy also depends on potential energy (due to particle spacing).
Example:
A metal rod is placed in contact with boiling water. After some time, the rod reaches a temperature of \(100^\circ \mathrm{C}\). Does this mean the rod has the same amount of heat as the boiling water? Explain.
▶️ Answer/Explanation
No, temperature and heat are different quantities. The rod having the same temperature as the boiling water means they are in thermal equilibrium — there is no net heat transfer between them. However, heat is the total energy due to the random motion of particles, which depends on mass, temperature, and specific heat capacity. Since the water has much greater mass and heat capacity, it contains more thermal energy than the rod.
Example:
Two cups of water are at \(25^\circ \mathrm{C}\), one containing 200 mL and the other 500 mL. Which one has the higher temperature? Which one has more thermal energy?
▶️ Answer/Explanation
Both cups have the same temperature: \(25^\circ \mathrm{C}\). Temperature is independent of the quantity of water. However, the 500 mL cup has more thermal energy because thermal energy depends on mass: \(Q = mc\Delta T\). A larger mass at the same temperature means more total internal energy.
Example:
A 1 kg block of copper at \(20^\circ \mathrm{C}\) is heated until it reaches \(80^\circ \mathrm{C}\). If the specific heat capacity of copper is \(390 \ \mathrm{J \, kg^{-1} \, ^\circ C^{-1}}\), calculate the heat energy supplied to the block and explain how this relates to internal energy.
▶️ Answer/Explanation
We use \( Q = mc\Delta T \):
\( Q = (1.0 \ \mathrm{kg}) (390 \ \mathrm{J \, kg^{-1} \, ^\circ C^{-1}}) (80 – 20)^\circ \mathrm{C} \)
\( Q = 390 \times 60 = 23{,}400 \ \mathrm{J} \)
This heat energy increases the internal energy of the copper block, which manifests as an increase in the average kinetic energy of its particles, raising the temperature from \(20^\circ \mathrm{C}\) to \(80^\circ \mathrm{C}\).
Final Answer: \(\boxed{Q = 23{,}400 \ \mathrm{J}}\)
