IB MYP 4-5 Physics- Thermometers and temperature measurement- Study Notes - New Syllabus
IB MYP 4-5 Physics-Thermometers and temperature measurement- Study Notes
Key Concepts
- Thermometers and temperature measurement
Thermometers and Temperature Measurement
Thermometers and Temperature Measurement
Temperature:
Temperature is a measure of the average kinetic energy of particles in a substance. It indicates how hot or cold an object is.
Principle of Temperature Measurement:
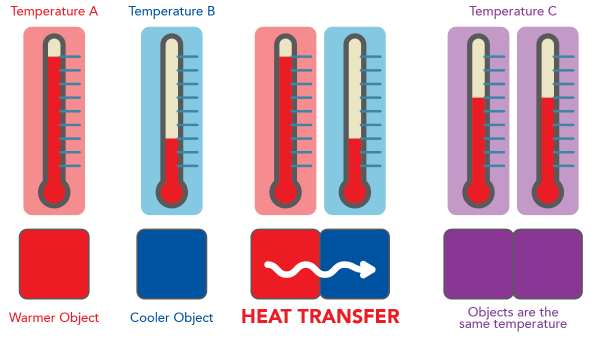
- Thermometers work based on a physical property that changes predictably with temperature (called a thermometric property).
- Examples of thermometric properties:
- Length or volume of a liquid (liquid-in-glass thermometers).
- Electrical resistance (resistance thermometers).
- Pressure of a fixed volume of gas (gas thermometers).
Common Types of Thermometers:
- Liquid-in-glass thermometers: Use mercury or alcohol; liquid expands/contracts with temperature.
- Bimetallic strip thermometers: Made of two metals with different expansion rates; bending indicates temperature change.
- Gas thermometers: Measure pressure change in a fixed volume of gas.
- Thermocouples: Use voltage difference between two metals; good for high-temperature ranges.
- Infrared thermometers: Measure emitted infrared radiation; useful for non-contact measurement.
Temperature Scales:
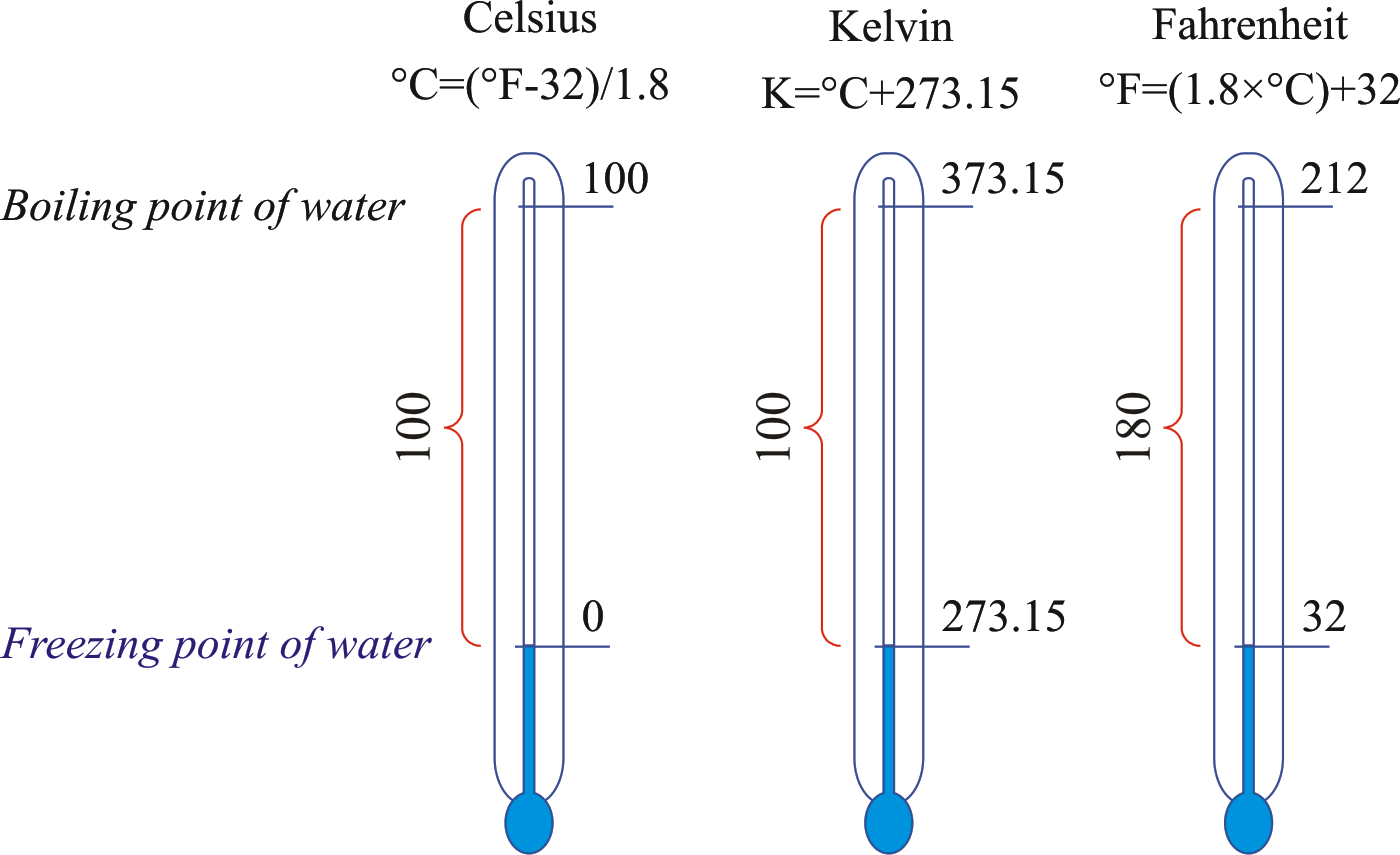
- Celsius scale (°C): Based on freezing point (0°C) and boiling point (100°C) of water at 1 atm pressure.
- Kelvin scale (K): Absolute temperature scale; 0 K is absolute zero, no negative values.
- Fahrenheit scale (°F): Used in some countries; water freezes at 32°F and boils at 212°F.
Converting between Temperature Scales:
T(K) = T(°C) + 273.15
T(°F) = \dfrac{9}{5} \times T(°C) + 32
T(°C) = \dfrac{5}{9} \times (T(°F) – 32)
Calibration of Thermometers:

- Calibration ensures readings are accurate.
- Uses fixed points:
- Lower fixed point: Temperature of pure melting ice at standard atmospheric pressure (0°C).
- Upper fixed point: Temperature of pure boiling water at standard atmospheric pressure (100°C).
- The distance between these two points is divided into equal parts to mark degrees.
Advantages and Disadvantages of Common Thermometers:
| Type | Advantages | Disadvantages |
|---|---|---|
| Mercury-in-glass | High accuracy, wide range | Toxic, cannot measure very low temperatures |
| Alcohol-in-glass | Safe, measures low temperatures | Evaporates easily, less precise |
| Thermocouple | Fast response, wide range | Requires calibration, less accurate for small temperature changes |
| Infrared thermometer | Non-contact, safe for hazardous environments | Expensive, affected by surface emissivity |
Example:
A Celsius thermometer reads 25°C. What is this temperature in Kelvin and Fahrenheit?
▶️ Answer/Explanation
Using T(K) = T(°C) + 273.15:
T(K) = 25 + 273.15 = \boxed{298.15 \ \text{K}}
Using T(°F) = \dfrac{9}{5} \times T(°C) + 32:
T(°F) = \dfrac{9}{5} \times 25 + 32 = \boxed{77°F}
Example:
A mercury-in-glass thermometer is calibrated such that the lower fixed point is 0°C and the upper fixed point is 100°C. If the mercury level is 40% of the distance from the lower to upper fixed point, what is the temperature?
▶️ Answer/Explanation
Temperature range = 100°C − 0°C = 100°C
40% of 100°C = 0.40 × 100 = \boxed{40°C}
Example:
A thermocouple shows a voltage of 2.5 mV. If its calibration chart says 1.0 mV corresponds to 25°C, what is the measured temperature?
▶️ Answer/Explanation
Temperature per mV = 25°C
Measured temperature = 2.5 × 25°C = \boxed{62.5°C}
