- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
A1.1.1 – Water as the Medium for Life
Essential Idea:
Water is the medium of life – without it, life as we know it would not exist. It plays a central role in nearly all biological processes, from the origin of life to the functioning of cells today.
🌊 1. The Origin of Life in Water
- Life is believed to have begun in water. The first cells likely formed in aquatic environments, where water supported essential chemical reactions.
- Even today, most biological processes occur in aqueous environments – within cells and throughout entire organisms.
💠 2. The Structure of Water
- Water (H₂O) is made of two hydrogen atoms covalently bonded to one oxygen atom.
- Oxygen is more electronegative and pulls shared electrons closer, making water a polar molecule:
- Oxygen side: Slightly negative (δ⁻)
- Hydrogen side: Slightly positive (δ⁺)
- This polarity allows hydrogen bonds to form between adjacent water molecules.
🧲 3. Hydrogen Bonding & Water’s Unique Properties
Hydrogen bonds form between water molecules – weak alone, but strong collectively – giving water life-supporting properties:
- Cohesion: Water sticks to itself (important in plant transport).
- Adhesion: Water sticks to surfaces (capillary movement).
- High specific heat: Water resists temperature change.
- High latent heat of vaporization: Evaporation cools organisms.
- Excellent solvent: Water dissolves many solutes due to polarity.
🌱 4. Why Water Is Essential for Life
a. Solvent for Biochemical Reactions
- Dissolves ions and molecules
- Enables enzyme-substrate interaction
- Supports transport of nutrients, gases, and wastes
b. Participates in Metabolic Reactions
- Hydrolysis: Breaks down molecules using water
- Condensation: Joins molecules and releases water
c. Thermal Buffer
- Water absorbs/releases heat slowly
- Helps maintain constant internal body temperature
d. Maintains Structure
- Stabilises proteins via hydrogen bonds
- Supports membrane structure (hydrophilic/hydrophobic zones)
- Helps hold DNA strands together
e. Supports Transport Systems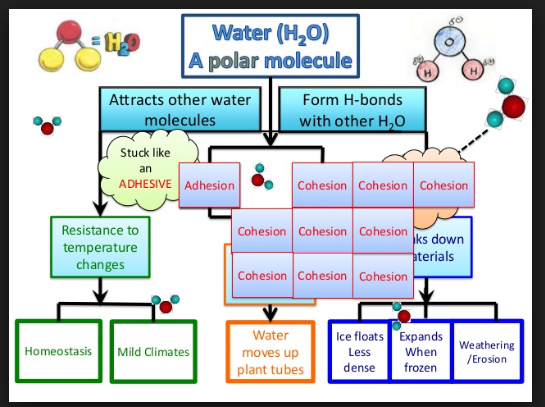
- Blood, lymph, cytoplasm, and plant sap are water-based
- Allows movement of nutrients, hormones, gases, and waste
🔬 5. Nature of Science: Theory Behind Hydrogen Bonds
Hydrogen bonding is a scientific theory explaining water’s observed behavior. Although we can’t directly see hydrogen bonds, the following features support their existence:
- High boiling point
- Surface tension
- High heat capacity
These consistent, observable outcomes validate the hydrogen bonding model – showcasing how theories explain natural phenomena.
a. Solvent for Biochemical Reactions
- Dissolves ions and molecules
- Enables enzyme-substrate interaction
- Supports transport of nutrients, gases, and wastes
🧬 Summary: Life Depends on Water Because…
- It is the medium where life originated
- It serves as a solvent, reactant, and temperature stabiliser
- It enables metabolic reactions and transport of substances
- It supports biological structure (DNA, proteins, membranes)
- Its remarkable properties arise from hydrogen bonding and polarity
A1.1.2 – Hydrogen Bonds from Polar Covalent Bonds in Water
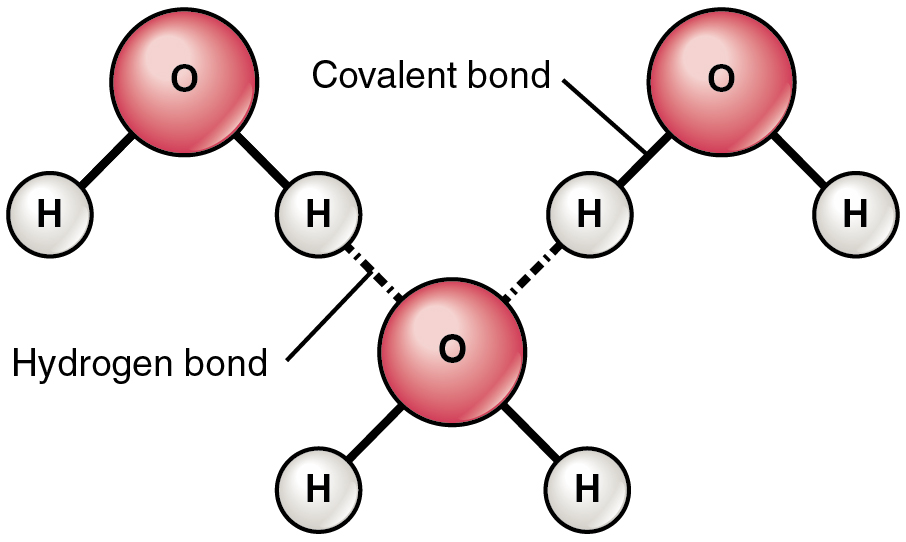
1. Water is a Polar Molecule
- Water (H₂O) = 1 oxygen + 2 hydrogen atoms
- Oxygen is more electronegative → pulls electrons closer
- Unequal sharing of electrons = polar covalent bonds
Oxygen → partial negative (δ⁻)
Hydrogen → partial positive (δ⁺)
2. Shape of Water
- Water has a bent shape
- This creates polarity – charge separation
- One side is slightly positive, the other slightly negative
3. Hydrogen Bonding
Hydrogen bonds form between:
- Slightly positive H of one water molecule
- Slightly negative O of another molecule
- Individually weak, but strong in large numbers
- Responsible for high boiling point, surface tension, etc.
4. Bonds in Water
| Type | Where it is Found |
|---|---|
| Polar covalent | Within one water molecule (O-H bond) |
| Hydrogen bond | Between two water molecules |
🔹Quick Definitions
- Atom: Smallest unit of matter (neutral)
- Ion: Atom with a charge
- Cation: Positive ion
- Anion: Negative ion
- Covalent bond: Sharing electrons
- Ionic bond: Transfer of electrons
- Hydrogen bond: Weak attraction between polar molecules
A1.1.3 – Cohesion of Water Molecules and Its Consequences for Organisms
1. What is Cohesion?
Cohesion refers to the tendency of water molecules to stick to each other. It is caused by hydrogen bonds between polar water molecules and gives water its internal “stickiness”.
Cohesion allows water to move as a continuous column through the xylem, even against gravity.
2. Role in Plants: Water Transport in Xylem
Water is pulled upward from roots to leaves by transpiration pull. Cohesive forces form an unbroken column in xylem vessels. These vessels are strengthened with lignin to withstand the tension created by water movement under negative pressure.
🔹 Steps in Water Movement Through Xylem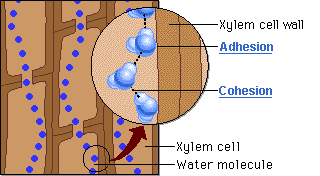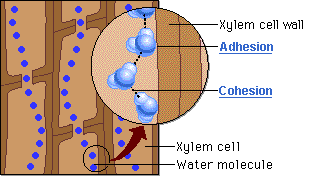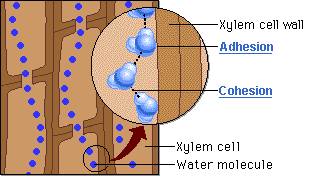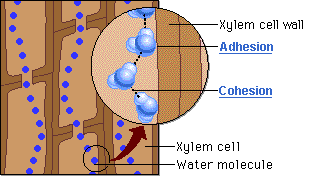
- Water evaporates from leaves via stomata (transpiration)
- This creates a negative pressure (tension) in the leaf
- Cohesion pulls the next water molecules upward as a chain
- Adhesion to xylem wall helps maintain the column
3. Surface Tension
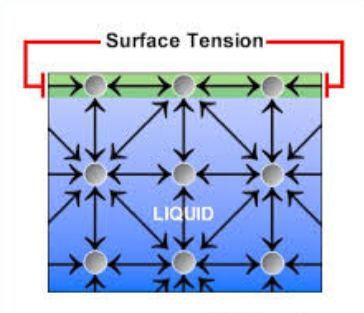
Surface tension arises because water molecules at the surface cohere more strongly to each other than to air. This creates a tight surface layer, like an elastic skin, due to inward hydrogen bond forces.
🔹 Benefits of Surface Tension
- Allows small insects like water striders to walk on water
- Creates surface habitats and supports floating organisms
- Helps in droplet formation and water movement through fine tubes
Cohesion vs Adhesion
| Property | Water sticks to… | Role in Plants |
|---|---|---|
| Cohesion | Other water molecules | Maintains water column in xylem |
| Adhesion | Surfaces (e.g., xylem wall) | Helps water climb up vessel walls |
A1.1.4 – Adhesion of Water and Its Impact on Organisms
1. What is Adhesion?
Adhesion refers to water molecules sticking to other polar or charged surfaces. It occurs due to hydrogen bonding between water and ionic/polar substances.
Adhesion helps water cling to plant cell walls and soil particles, aiding transport.
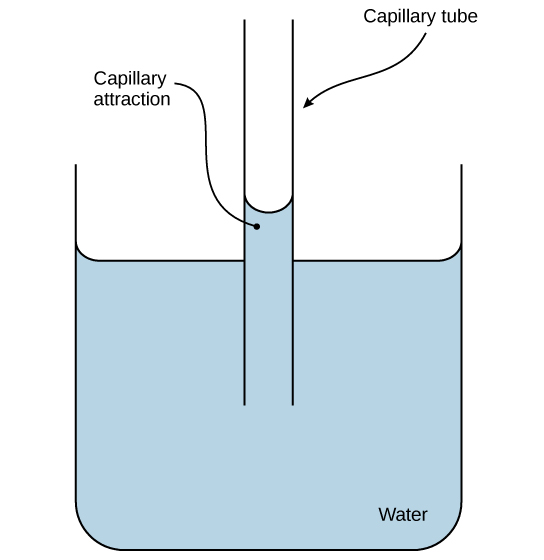
2. Role in Capillary Action
Capillary action occurs when water rises in narrow spaces against gravity. This happens when adhesion to the surface is stronger than the cohesion between water molecules.
- Glass tubes
- Soil pores
- Xylem vessels and plant cell walls
3. Capillary Action in Plants
Xylem walls are slightly charged, attracting water molecules. Adhesion allows water to stick to the xylem vessels and move upward. It works with cohesion to support the transpiration pull. This also allows water transport before leaves develop (e.g., early spring).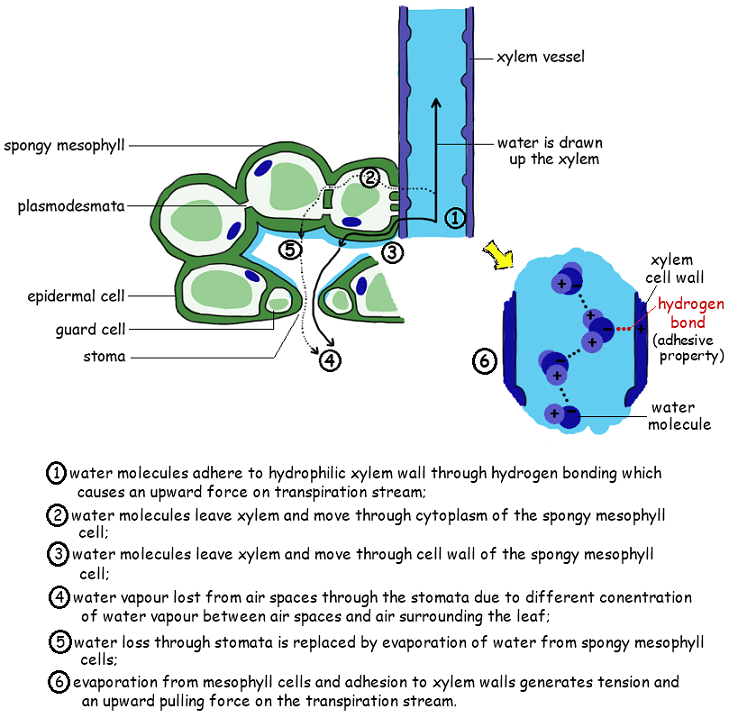
4. Capillary Action in Soil
Water rises through narrow soil pores via capillary action. This enables plants to draw moisture from near the surface, especially during dry periods or at early growth stages.
🔹 Summary Table
| Process | Description | Benefit to Plants |
|---|---|---|
| Adhesion | Water sticks to xylem and cell walls | Maintains water flow in xylem |
| Capillary action | Water rises in narrow spaces due to adhesion | Moves water in roots, stems, and soil |
| Transpiration pull | Evaporation-driven, adhesion helps maintain the column | Ensures steady water supply to leaves |
A1.1.5 – Solvent Properties of Water and Its Role in Metabolism and Transport
1. Water as a Solvent
Water is a polar molecule, meaning it has partial positive and negative charges. This allows it to surround and dissolve many substances, making it known as the universal solvent.
Water acts as a medium for metabolic reactions and helps transport essential substances in both plants and animals.
2. Hydrophilic vs Hydrophobic
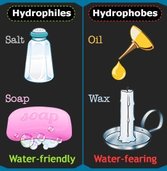
| Type | Meaning | Example |
|---|---|---|
| Hydrophilic | Water-loving, dissolves in water | Ions, sugars, amino acids |
| Hydrophobic | Water-hating, does not dissolve in water | Lipids, fats, oils |
Hydrophilic molecules interact easily with water, while hydrophobic molecules cluster away from water. This is essential in forming cell membranes (e.g., phospholipid bilayers).
3. Role in Metabolism
Most biochemical reactions occur in water because:
- Enzymes function best in aqueous (watery) solutions
- Water helps transport reactants and products
- Molecules dissolve and collide more easily in water
4. Transport in Living Organisms
Water dissolves essential substances and enables easy transport:
- In plants: Carries mineral ions in xylem and sugars in phloem (sap)
- In animals: Blood plasma (mostly water) transports oxygen, glucose, hormones, and waste products
5. Summary of Water’s Solvent Role
| Function | Example |
|---|---|
| Medium for metabolism | Enzymes work in cytoplasm (aqueous) |
| Transport in plants | Ions in xylem, sugars in phloem |
| Transport in animals | Plasma carries nutrients, gases, hormones |
| Cell structure | Hydrophobic molecules form membranes |
A1.1.6 – Physical Properties of Water and Their Consequences for Aquatic Animals
1. Key Physical Properties of Water
| Property | Cause/Definition | Consequence for Aquatic Life |
|---|---|---|
| Buoyancy | Upward force on objects in fluid; opposes gravity | Helps animals like seals and loons float with less effort |
| Viscosity | Friction between water molecules due to hydrogen bonds | Requires more energy to swim → streamlining is needed |
| Thermal conductivity | Water transfers heat quickly (faster than air) | Aquatic animals lose heat quickly → need insulation (blubber) |
| Specific heat capacity | Water absorbs/releases heat slowly due to hydrogen bonding | Stable temperature in water habitats |
Aquatic environments offer support and temperature stability but require energy for swimming and insulation against heat loss.
2. Water vs Air – Key Differences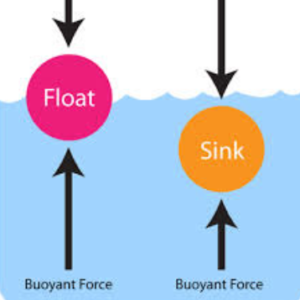
| Feature | Water | Air |
|---|---|---|
| Density / Buoyancy | High – provides support | Low – animals must support body weight |
| Viscosity | High – causes drag | Low – easy movement |
| Thermal conductivity | High – absorbs heat rapidly | Low – less heat loss |
| Specific heat | High – slow temperature changes | Low – temperature changes quickly |
3. Organism Examples
🔸 Ringed Seal (Pusa hispida)

- Floats easily in water due to buoyancy
- Faces drag while swimming → streamlined body
- Loses heat fast due to water’s high thermal conductivity
- Has thick blubber to insulate and retain heat
- Lives in a thermally stable habitat due to high specific heat of water
🔸 Black-Throated Loon (Gavia arctica)

- Buoyancy helps it float on water
- Must use more energy to fly (less support in air)
- Air’s low viscosity makes flying easier
- Loses less body heat in air due to low thermal conductivity
- Must adjust to air’s quick temperature changes (low specific heat)
4. Summary of Adaptations
| Physical Property | Ringed Seal | Black-Throated Loon |
|---|---|---|
| Buoyancy | Floats easily | Floats in water, not in air |
| Viscosity | Streamlined to reduce drag | Streamlined for flying and swimming |
| Thermal conductivity | Needs blubber to retain heat | Loses less heat; no blubber needed |
| Specific heat | Stable habitat | Air temp changes → more thermoregulation |
Additional Higher Level
A1.1.7 – Extraplanetary Origin of Water and Its Retention on Earth
1. Where Did Earth’s Water Come From?
Hypothesis: Water was delivered to early Earth by asteroids and comets carrying frozen water.
- These collisions occurred over 4 billion years ago
- Geological evidence shows liquid water on Earth around 4.4 billion years ago
2. Why Did Earth Keep Its Water?
| Factor | Explanation |
|---|---|
| Earth’s gravity | Strong enough to retain water vapour in the atmosphere |
| Earth’s temperature | Just right to allow water to condense and stay liquid |
3. Water’s Role in the Evolution of Life

- Universal solvent for key biochemical reactions
- Supports stable thermal conditions and surface tension
- Vital for enzyme activity, metabolic reactions, and cell structure
4. Water and the Search for Life in Space
Scientists prioritize the search for water when looking for extraterrestrial life.
- Goldilocks Zone: Region where conditions are “just right” for liquid water
- Planets/moons like Europa and ancient Mars show signs of water presence
- Liquid water = key requirement for life as we know it.
A1.1.8 – Water and the Search for Extraterrestrial Life
1. Why is Water Central to the Search for Life?
All known life depends on water for survival – it supports metabolism, transport of molecules, and thermal regulation.
- Water allows biochemical reactions to occur
- Its polarity and solvent properties make it ideal for life processes
- Liquid water is a strong indicator of habitability
If we find liquid water on another planet, it significantly raises the chances of finding life there.
2. The “Goldilocks Zone”
Also known as the habitable zone – the region around a star where liquid water can exist.
- If a planet is too close: water evaporates
- If it’s too far: water freezes
- Only within this zone can stable liquid water exist
3. Importance of the Goldilocks Zone
Scientists use this idea to explore:
- Mars – had surface water in the past
- Europa (moon of Jupiter) – likely has a subsurface ocean
- Exoplanets – planets in other solar systems with similar distances from their stars
4. Summary
| Concept | Importance for Life |
|---|---|
| Liquid water | Required for metabolism, transport, enzyme activity |
| Goldilocks zone | Allows liquid water to exist – essential for life |
| Water = Priority | First thing scientists look for when searching for alien life |
