- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
B1.1.1 – Chemical Properties of Carbon That Enable Life’s Diversity
🧪 Why Carbon? The Backbone of Life

Carbon is the foundation of all organic molecules, and its unique chemical properties make it the most versatile element in biology. Without carbon’s flexibility, the molecular complexity of life would not exist.
🔬 Covalent Bonding: Carbon’s Secret Power

Carbon has 4 valence electrons, allowing it to form four covalent bonds. These bonds can be:
- Single (–C–C–)
- Double (–C=C–)
- Triple (–C≡C–)
✅ This bonding versatility allows carbon to build stable, complex, and diverse molecules.
🧬 Types of Carbon-Based Structures in Life
1. Straight (Unbranched) Chains: Continuous chains of carbon atoms
Example: Fatty acids (e.g., palmitic acid: CH₃–(CH₂)₁₄–COOH)
2. Branched Chains: Carbon chains that branch off the main chain

Example: Glycogen (a storage polysaccharide made of branched glucose chains)
3. Ring Structures: Carbon atoms form closed loops or rings
Example: Glucose (6-membered ring sugar), Cholesterol (4-ringed steroid)
🧠 Note: Carbon can form single or multiple rings, and even aromatic rings (like benzene).
🧪 Examples of Molecular Diversity from Carbon
| Molecule | Structure Type | Key Features |
|---|---|---|
| Glucose | Ring | Energy-rich, 6-carbon sugar |
| Fatty acid | Unbranched chain | Long hydrocarbon tail; part of lipids |
| Glycogen | Branched with rings | Highly branched polysaccharide for energy storage |
| DNA bases | Single/double rings | Nitrogenous bases (A, T, C, G) made of carbon rings |
| Cholesterol | Multi-ring structure | Steroid with 4 fused carbon rings |
Fatty acid: a linear molecule

Glucose: a ringed molecule

Glycogen: a branched molecule with multiple rings

📏 Nature of Science (NOS): Scientific Conventions
Scientific communication depends on globally accepted standards. Measurement prefixes are used consistently in chemistry and biology.
| Prefix | Symbol | Meaning |
|---|---|---|
| kilo | k | 10³ (1,000) |
| centi | c | 10⁻² (0.01) |
| milli | m | 10⁻³ (0.001) |
| micro | µ | 10⁻⁶ (0.000001) |
| nano | n | 10⁻⁹ (0.000000001) |
🧠 Summary Box – Why Carbon Makes Life Possible
4 covalent bonds: Allows carbon to form stable, complex molecules
Self-bonding: Enables chains, rings, and branches
Multiple bond types: Adds versatility to molecular design
Molecular diversity: Essential for all biological macromolecules (carbohydrates, lipids, proteins, nucleic acids)
B1.1.2 – Formation of Macromolecules by Condensation Reactions
🧪 What Are Macromolecules?
Macromolecules (also called polymers) are large biological molecules made by joining smaller units called monomers.
Monomer: A small, repeating unit (e.g., glucose, amino acid, nucleotide)
Polymer: A long chain of monomers linked by chemical bonds (e.g., starch, protein, DNA)
💧 Condensation Reaction: How Monomers Join
A condensation reaction (also called dehydration synthesis) is a chemical reaction in which two monomers bond by removing water.
⚙️ What Happens?
One monomer loses a –OH (hydroxyl) group.
The other monomer loses an –H (hydrogen).
The removed H₂O (water) is released.
A new covalent bond forms between the monomers.
🧠 This is the opposite of hydrolysis, where water is added to break bonds.
🔬 Macromolecules Formed by Condensation Reactions
| Macromolecule | Monomers | Bond Formed | Example |
|---|---|---|---|
| Polysaccharides | Monosaccharides (e.g. glucose) | Glycosidic bond | Starch, cellulose, glycogen |
| Polypeptides | Amino acids | Peptide bond | Enzymes, keratin, hemoglobin |
| Nucleic acids | Nucleotides | Phosphodiester bond | DNA, RNA |
1. Polysaccharides (Carbohydrate Polymers)
- Condensation of monosaccharides (like glucose) produces polysaccharides.
- Example: Glucose + Glucose → Maltose + Water
- Repeated condensation reactions form starch (plant energy storage).
- Glycosidic bonds link sugar units.
2. Polypeptides (Protein Chains)
- Condensation of amino acids produces polypeptides (proteins).
Each amino acid has:- An amino group (–NH₂)
- A carboxyl group (–COOH)
- Peptide bond forms between –COOH and –NH₂ with release of water.
- Example: Amino acid + Amino acid → Dipeptide + Water → Polypeptide
3. Nucleic Acids (DNA & RNA)
Condensation of nucleotides forms DNA or RNA strands.
Each nucleotide contains:
- A phosphate group
- A sugar (deoxyribose or ribose)
- A nitrogenous base (A, T/U, G, C)
Phosphodiester bonds form between phosphate of one nucleotide and sugar of the next, releasing water.
🧠 Summary Box – Condensation Reactions in Macromolecules
| Process | Monomer | Polymer | Bond Formed | Water Released? |
|---|---|---|---|---|
| Making starch or glycogen | Glucose | Polysaccharide | Glycosidic bond | Yes |
| Making proteins | Amino acids | Polypeptides | Peptide bond | Yes |
| Making DNA or RNA | Nucleotides | Nucleic acids | Phosphodiester bond | Yes |
B1.1.3 – Digestion of Polymers into Monomers by Hydrolysis Reactions
💧 What Is Hydrolysis?
A polymer is split into smaller units.
Water breaks into –H and –OH.
These attach to the resulting monomers to stabilize them.
- Hydrolysis is the chemical breakdown of polymers into monomers using water.
- The term comes from “hydro” (water) and “lysis” (to break).
- It’s the reverse of a condensation (dehydration) reaction.
- A water molecule is added, and its components (–H and –OH) are used to break covalent bonds between monomers.
🧠 Hydrolysis is vital in digestion – breaking down food macromolecules into absorbable units.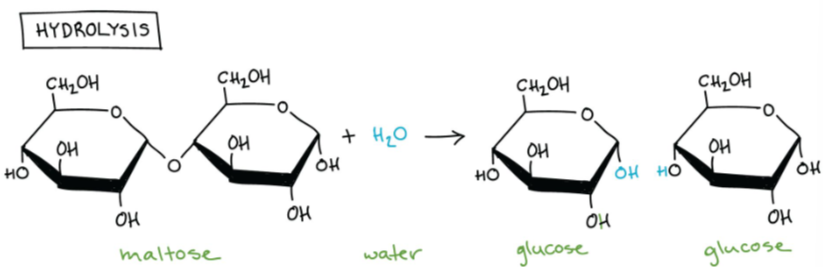
🍽️ Examples of Hydrolysis in the Human Body
| Polymer | Monomer Produced | Enzyme Involved | Bond Broken |
|---|---|---|---|
| Starch (polysaccharide) | Glucose | Amylase | Glycosidic bond |
| Protein (polypeptide) | Amino acids | Pepsin, Trypsin | Peptide bond |
| DNA/RNA (nucleic acid) | Nucleotides | Nuclease enzymes | Phosphodiester bond |
| Lipids (triglycerides) | Glycerol + Fatty acids | Lipase | Ester bonds |
🧪 Hydrolysis vs Condensation
| Feature | Condensation Reaction | Hydrolysis Reaction |
|---|---|---|
| Water involvement | Water is released | Water is used/added |
| Purpose | Builds polymers from monomers | Breaks polymers into monomers |
| Bond effect | Forms covalent bonds | Breaks covalent bonds |
| Biological role | Anabolic (building) | Catabolic (breaking down, digestion) |
🧠 Summary Box – Hydrolysis: Breaking Down to Build Up
Hydrolysis reactions split macromolecules into their basic monomers using water.
These reactions are crucial for:
- Digesting food
- Cellular metabolism
- Nutrient absorption
Every major biological macromolecule (carbs, proteins, nucleic acids, lipids) can be broken down by hydrolysis.
💧 “Hydrolysis is how life takes big bites and turns them into usable parts.”
B1.1.4 – Form and Function of Monosaccharides
🍬 What Are Monosaccharides?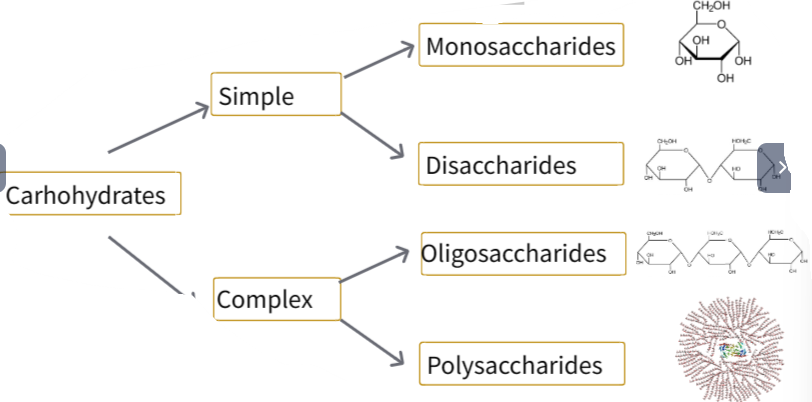
Monosaccharides are the simplest carbohydrates – single sugar units that cannot be hydrolyzed into smaller sugars.
General formula: (CH₂O)n, where n = 3 to 7
Composed of carbon (C), hydrogen (H), and oxygen (O)
In aqueous solutions, they mostly exist in ring structures.
🔎 Types of Monosaccharides by Carbon Number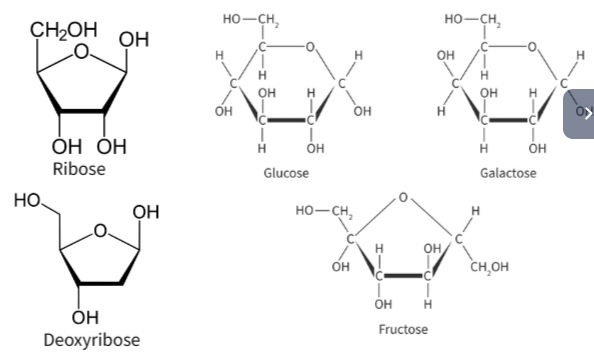
| Type | Carbon Atoms | Examples | Biological Function |
|---|---|---|---|
| Pentoses | 5 | Ribose, Deoxyribose | Found in RNA and DNA (nucleic acids) |
| Hexoses | 6 | Glucose, Fructose, Galactose | Energy sources, precursors to polysaccharides |
🔬 Structure of Glucose
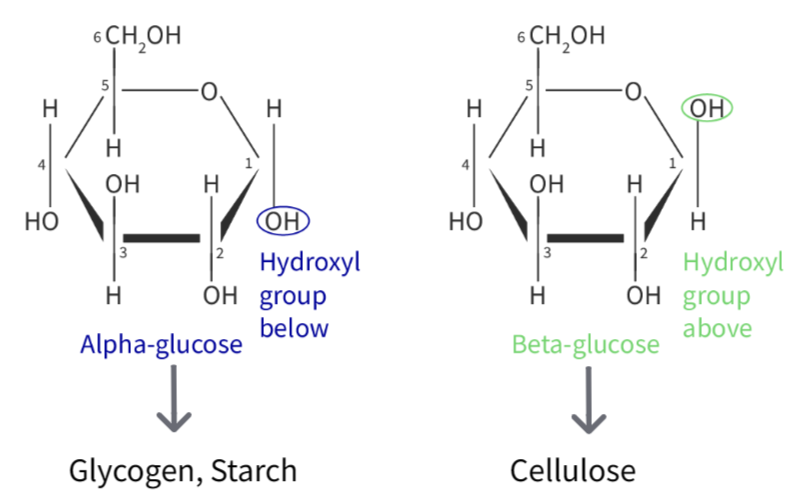
Glucose has the molecular formula C₆H₁₂O₆ and contains multiple hydroxyl (–OH) groups, making it polar and soluble.
It exists in two isomeric ring forms:
- α-glucose: –OH on carbon 1 is below the ring plane

- β-glucose: –OH on carbon 1 is above the ring plane
💧 Solubility and Transport
Due to the presence of polar –OH groups, glucose dissolves easily in water.
This enables glucose to be transported efficiently in:
- Animal bloodstream
- Plant phloem sap
Chemical Stability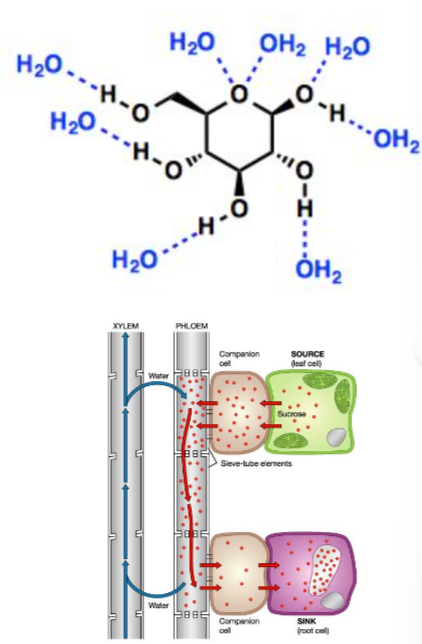
The ring structure of glucose minimizes repulsion between hydroxyl groups, making the molecule chemically stable.
This allows glucose to serve as a building block in polymers like cellulose, glycogen, and starch.
🔋 Oxidation and Energy Yield
Glucose is a key energy source in cellular respiration:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ATP
This aerobic process releases large amounts of energy for cellular work.
🧠 Summary Box – Why Monosaccharides Matter
| Feature | Why It’s Important |
|---|---|
| Small & soluble | Easily moves through biological fluids (e.g., blood, sap) |
| Energy source | Releases ATP when oxidized |
| Structural use | Ribose/deoxyribose in nucleic acids |
| Polymer precursor | Forms starch, glycogen, cellulose |
| Isomeric flexibility | Enables specific biological roles (e.g., α- vs β-glucose) |
B1.1.5 – Polysaccharides as Energy Storage Compounds
🍞 What Are Polysaccharides?
Polysaccharides are large carbohydrate polymers made by linking many alpha-glucose monomers via condensation reactions.
They are ideal for energy storage due to their:
- Compact shape
- Insolubility in water
- Ease of breakdown when energy is needed
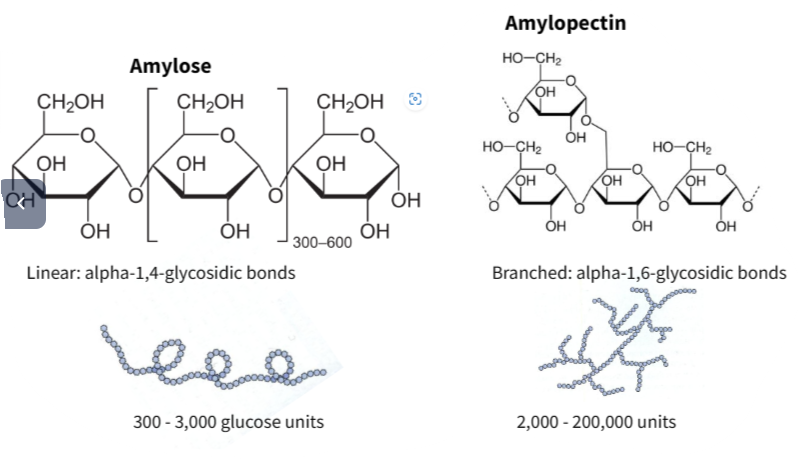
🌱 Starch: Energy Storage in Plants
Starch is made of two molecules – amylose and amylopectin – and is stored in chloroplasts and storage organs such as tubers and seeds.
| Component | Structure | Function |
|---|---|---|
| Amylose | Unbranched helix of α-glucose | Compact for storage |
| Amylopectin | Branched chain of α-glucose | Allows rapid hydrolysis when energy is needed |
🌀 Amylose coils into a helical shape – this makes it space-efficient.
🌿 Starch is insoluble, so it does not affect the osmotic balance in plant cells.
🐾 Glycogen: Energy Storage in Animals
Glycogen is the animal equivalent of starch, stored mainly in liver and muscle cells.
- Made of α-glucose like starch
- Highly branched – more than amylopectin (branches every 8–12 units)
- Allows rapid energy release when needed (e.g., during exercise)
📌 Its compact structure allows dense energy storage in cells without occupying too much space.
🔄 Adding & Removing Glucose Units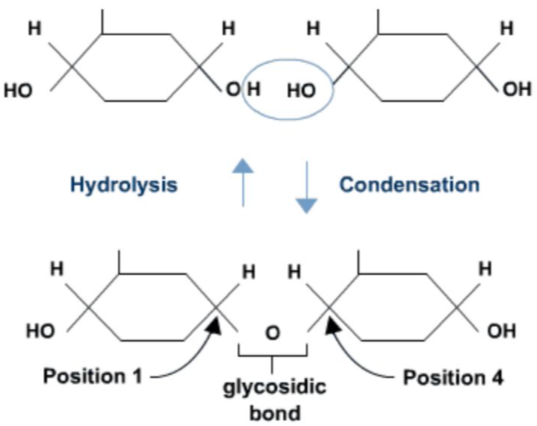
| Process | Reaction Type | Function |
|---|---|---|
| Energy storage | Condensation | Adds α-glucose units to grow the chain |
| Energy release | Hydrolysis | Breaks glycosidic bonds to release glucose |
Enzymes regulate both processes in plants and animals, enabling quick energy mobilization when needed.
🧠 Summary Box – Polysaccharides as Energy Stores
| Feature | Biological Advantage |
|---|---|
| Coiled & branched structure | Compact, fits large energy store in small space |
| Insoluble in water | Prevents osmosis-related swelling in cells |
| Easily hydrolyzed | Rapid glucose release for respiration |
| Made from α-glucose | Uniform subunits → efficient synthesis and degradation |
| Branched (esp. glycogen) | Multiple ends for enzyme access → fast mobilization of energy |
B1.1.6 – Structure of Cellulose and Its Role as a Structural Polysaccharide
🌿 What Is Cellulose?
- Cellulose is a structural polysaccharide made of β-glucose monomers. It is a major component of plant cell walls.
- Unlike starch or glycogen (made of α-glucose), cellulose is composed of β-glucose units.
- The orientation of glucose molecules alternates – every second monomer is flipped 180°.
🧱 Structure of Cellulose
| Structural Feature | Functional Outcome |
|---|---|
| Alternating β-glucose orientation | Forms straight, unbranched chains |
| Chains lie parallel | Allows tight packing into bundles (microfibrils) |
| Hydrogen bonding between chains | Provides rigidity and tensile strength |
| Insolubility in water | Maintains structural integrity in aqueous environments |
🪵 Function of Cellulose in Plants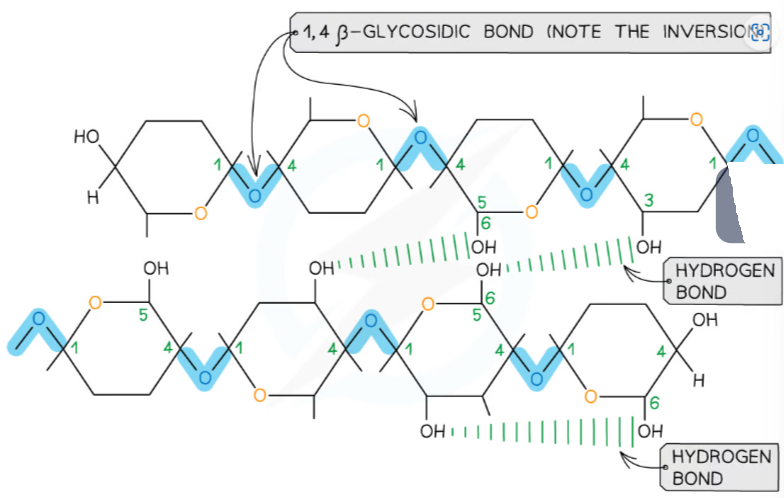
- Forms the main component of plant cell walls
- Provides mechanical strength and structural support
- Prevents cells from bursting due to osmotic intake of water
- Maintains shape and rigidity in leaves, stems, and other organs
🧠 Summary Box – Why Cellulose Matters
| Feature | Why It’s Important in Plants |
|---|---|
| β-glucose polymer | Enables straight, rigid structure |
| Hydrogen-bonded microfibrils | Increases strength and resistance to stretching |
| Insoluble and stable | Ideal for building long-lasting plant cell walls |
| Unbranched chains | Maximizes surface contact for hydrogen bonding |
B1.1.7 – Role of Glycoproteins in Cell-Cell Recognition
🧬 What Are Glycoproteins?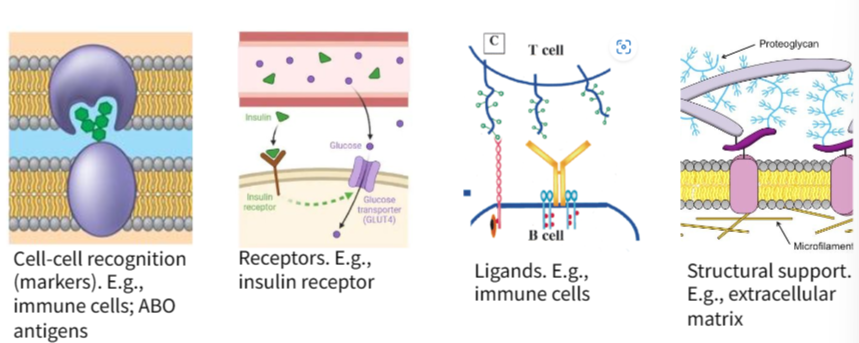
Glycoproteins are proteins with carbohydrate chains covalently attached. They are typically found embedded in the cell membrane.
The carbohydrate part extends outside the cell and is involved in essential processes like communication, immune defense, and recognition.
🔍 Functions of Glycoproteins in Cell–Cell Recognition
| Function | Explanation |
|---|---|
| Cell identification | Helps cells distinguish self from non-self |
| Immune response | Enables detection of pathogens by the immune system |
| Tissue compatibility | Crucial in organ transplants and avoiding rejection |
| Pathogen interaction | Used by viruses and bacteria to attach to host cells |
🩸 ABO Blood Group System – A Key Example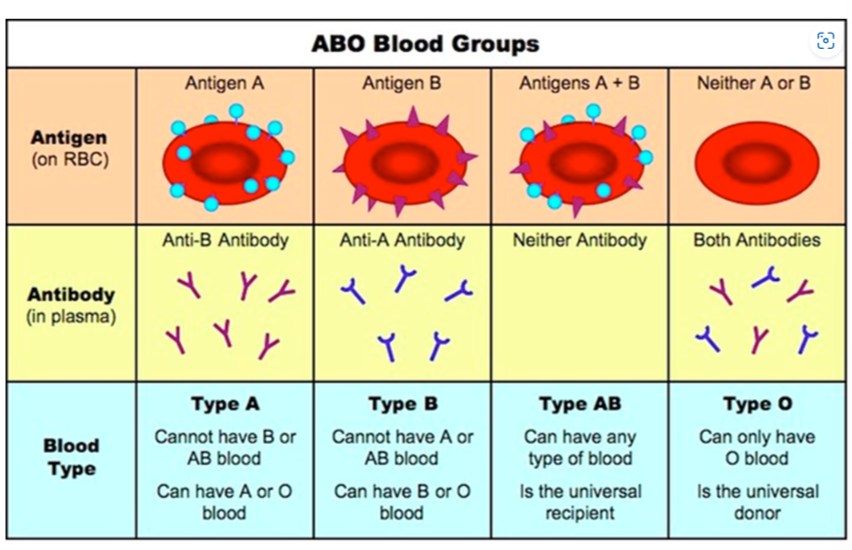
The ABO blood group system is based on the presence of specific glycoproteins (antigens) on red blood cells.
| Blood Group | Glycoprotein (Antigen) | Recognized as |
|---|---|---|
| A | A antigen (A sugar chain) | “Self” for A-type individuals |
| B | B antigen (B sugar chain) | “Self” for B-type individuals |
| AB | Both A and B antigens | Universal recipient |
| O | No A/B antigens | Universal donor (basic backbone only) |
🧠 Summary Box – Why Glycoproteins Matter
| Feature | Why It’s Important |
|---|---|
| Protein + carbohydrate | Combines stability with surface signaling function |
| Involved in immunity | Helps detect pathogens and foreign cells |
| Determines blood type | ABO antigens are glycoproteins that can trigger immune responses |
| Enables communication | Facilitates cell–cell signaling in tissues and organs |
B1.1.8 – Hydrophobic Properties of Lipids
🧪 What Are Lipids?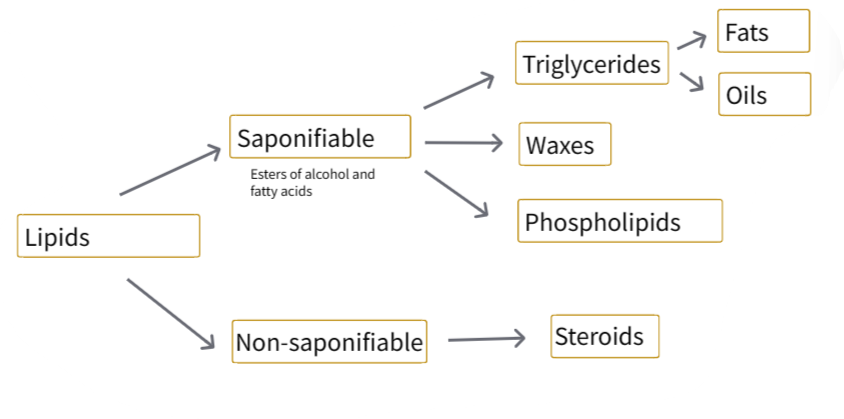
Lipids are non-polar biological molecules that are hydrophobic – they do not dissolve in water.
They include fats, oils, waxes, and steroids. Lipids are mainly composed of carbon (C), hydrogen (H), and a small amount of oxygen (O).
They are insoluble in water but dissolve in non-polar solvents like ethanol, ether, or chloroform.
🌊 Hydrophobic Nature Explained
| Property | Explanation |
|---|---|
| Non-polar structure | Lipids lack charged regions, so water molecules are not attracted to them |
| No hydrogen bonding | Water cannot form hydrogen bonds with lipid molecules |
| Tendency to cluster | Lipids clump together to minimize surface area exposed to water |
🧴 Examples of Lipids and Their Forms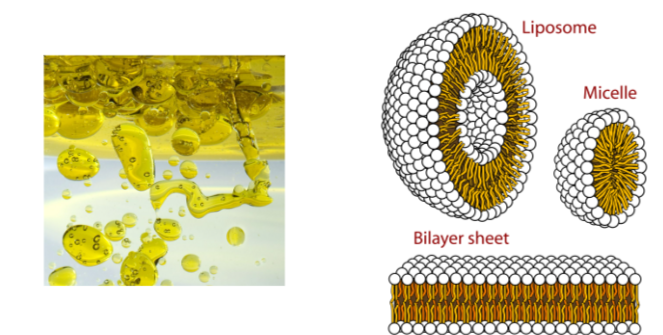
| Type of Lipid | Examples | Physical State at Room Temperature |
|---|---|---|
| Fats | Butter, animal fat | Solid (higher melting point) |
| Oils | Olive oil, sunflower oil | Liquid (lower melting point) |
| Waxes | Beeswax, leaf cuticle wax | Solid, used as waterproof coating |
| Steroids | Cholesterol, hormones | Involved in signaling and membrane structure |
🌱 Biological Significance of Lipid Hydrophobicity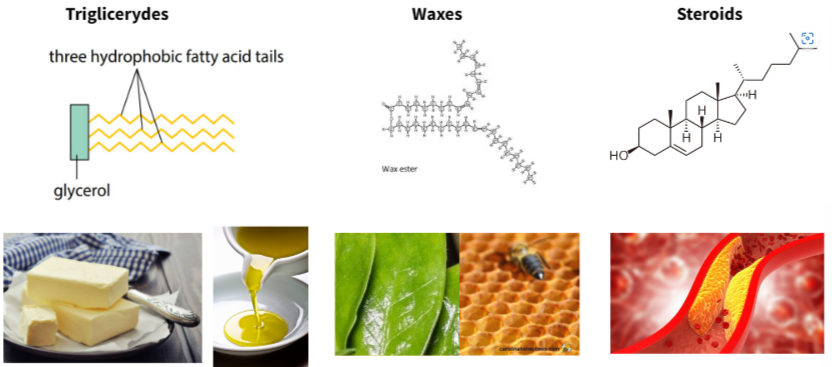
| Biological Function | Hydrophobic Role |
|---|---|
| Cell membranes (phospholipids) | Form water-repelling barriers that compartmentalize the cell |
| Energy storage (fats, oils) | Store large amounts of energy without water interference |
| Waterproofing (waxes) | Create protective barriers on leaves, feathers, and fur |
| Hormone function (steroids) | Diffuse through membranes to regulate processes |
🧠 Summary Box – Hydrophobic Nature of Lipids
| Feature | Why It’s Important |
|---|---|
| Insoluble in water | Helps form membranes and waterproof barriers |
| Soluble in organic solvents | Enables lipid-based storage and signaling roles |
| Clump together in water | Forms droplets, vesicles, or bilayers that organize cellular boundaries |
B1.1.9 – Formation of Triglycerides and Phospholipids by Condensation Reactions
🧪 What Are Lipids Made Of?
Lipids such as triglycerides and phospholipids are formed through condensation reactions – a process where molecules are joined and water is released.
Glycerol: A 3-carbon alcohol with –OH groups
Fatty acids: Long hydrocarbon chains with a terminal –COOH group
Phosphate group: Found in phospholipids and attached to glycerol
🔗 Triglycerides – Energy Storage Lipids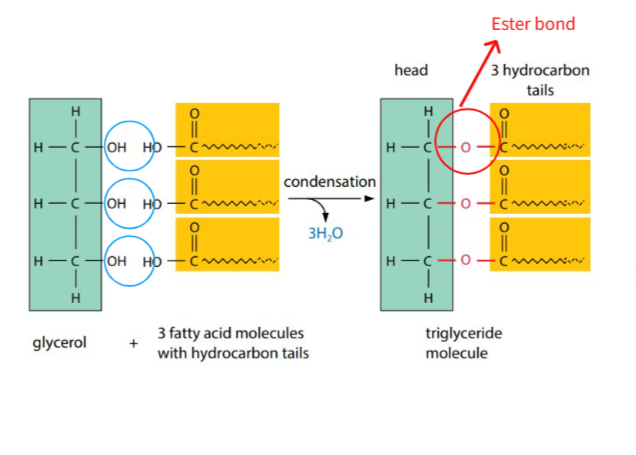
| Component | Number in Triglyceride |
|---|---|
| Glycerol | 1 |
| Fatty acids | 3 |
Each fatty acid forms an ester bond with glycerol via a condensation reaction, releasing one water molecule per bond.
Total: 3 ester bonds → 3 water molecules released
Triglycerides are non-polar and hydrophobic. They are used for long-term energy storage, insulation, and protection.
🧱 Phospholipids – Membrane-Forming Lipids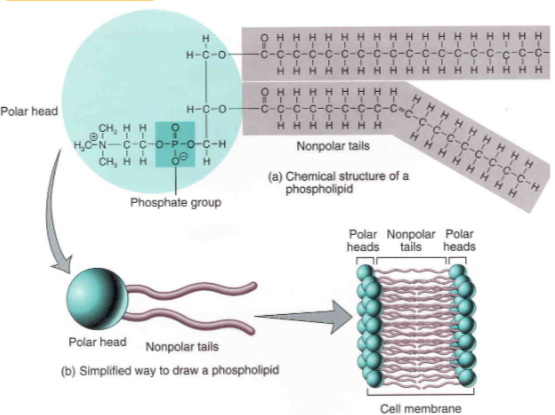
| Component | Number in Phospholipid |
|---|---|
| Glycerol | 1 |
| Fatty acids | 2 |
| Phosphate group | 1 |
Phospholipids are amphipathic – their phosphate head is hydrophilic, while fatty acid tails are hydrophobic.
This dual nature allows them to form bilayers in membranes, with tails inward and heads facing water.
🧠 Summary Box – Formation of Lipids via Condensation
| Lipid Type | Key Components | Function |
|---|---|---|
| Triglyceride | 1 glycerol + 3 fatty acids | Energy storage, insulation |
| Phospholipid | 1 glycerol + 2 fatty acids + 1 phosphate | Forms membranes (bilayers) |
| Bond Type | Ester bonds via condensation reactions | |
| Water Released | One per ester bond (3 for triglycerides) | |
B1.1.10 – Differences Between Saturated, Monounsaturated, and Polyunsaturated Fatty Acids
🧬 What Are Fatty Acids?
Fatty acids are long hydrocarbon chains with a carboxylic acid group (–COOH) at one end. They are the building blocks of triglycerides and phospholipids.
They differ based on:
– The length of the carbon chain
– The number of carbon-carbon double bonds (C=C)
🧪 Types of Fatty Acids
| Type | C=C Double Bonds | Structure | Melting Point | State at Room Temp |
|---|---|---|---|---|
| Saturated | 0 | Straight chains | High | Solid (e.g. butter) |
| Monounsaturated | 1 | One kink | Lower than saturated | Liquid/semi-solid |
| Polyunsaturated | 2 or more | Multiple bends | Lowest | Liquid (e.g. oils) |
🔥 Effect of Double Bonds on Melting Point
C=C double bonds introduce kinks in the fatty acid chain, which prevent molecules from packing closely. This reduces intermolecular forces and lowers melting points.
Saturated fats: pack tightly → solid
Unsaturated fats: loosely packed → liquid
🌿 Sources and Biological Roles
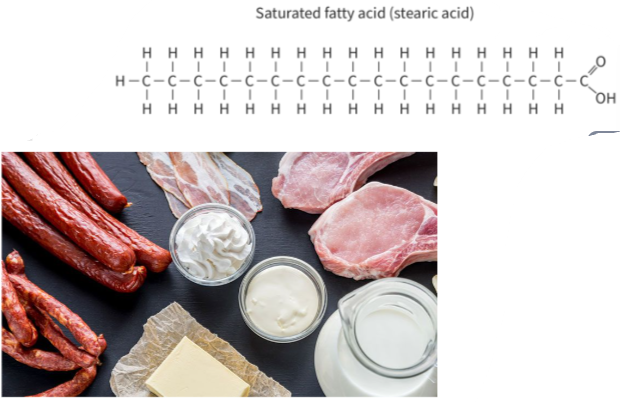

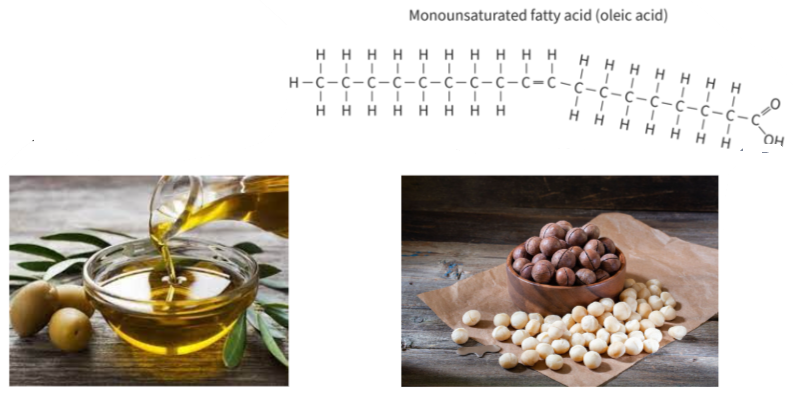
| Fatty Acid Type | Common Sources | Biological Use |
|---|---|---|
| Saturated | Animal fats, butter, coconut oil | Energy storage in warm-blooded animals |
| Monounsaturated | Olive oil, nuts | Healthy fats, support membrane fluidity |
| Polyunsaturated | Fish oils, plant oils (omega-3s, linoleic acid) | Heart and brain function, plant energy storage |
🧠 Summary Box – Comparing Fatty Acids
| Feature | Saturated | Monounsaturated | Polyunsaturated |
|---|---|---|---|
| C=C double bonds | 0 | 1 | 2 or more |
| Shape | Straight | One kink | Multiple kinks |
| Melting point | Highest | Moderate | Lowest |
| State at room temp | Solid | Semi-solid/Liquid | Liquid |
| Major sources | Animal fats, butter | Olive oil, nuts | Fish oils, vegetable oils |
B1.1.11 – Triglycerides in Adipose Tissues for Energy Storage and Thermal Insulation
🧪 What Are Triglycerides?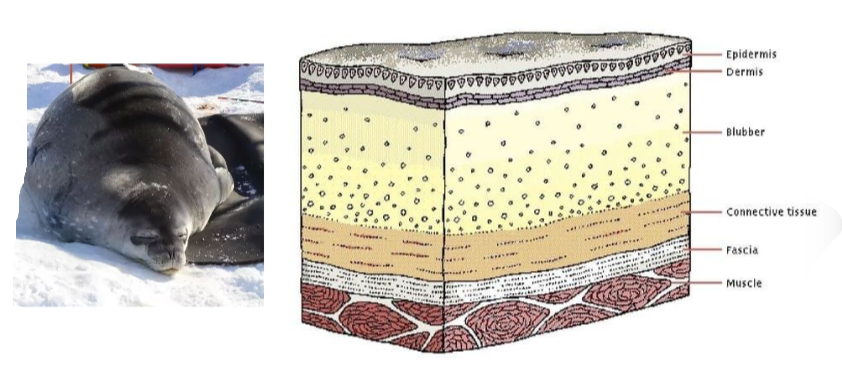
Triglycerides are lipids made of one glycerol molecule joined to three fatty acid chains via condensation reactions.
They are stored in adipose tissue beneath the skin and around internal organs, making them:
– Energy-rich
– Hydrophobic
– Chemically stable
🔋 Function 1: Long-Term Energy Storage

| Why Triglycerides Are Ideal for Storage | Explanation |
|---|---|
| High energy content | Release more energy per gram than carbohydrates |
| Compact storage | Hydrophobic nature excludes water → saves space |
| Non-reactive | Chemically stable and safe to store long-term |
| Lightweight | Useful for mobility in animals (e.g., birds) |
Triglycerides accumulate when energy intake exceeds use and are broken down via hydrolysis during energy demand.
🌡️ Function 2: Thermal Insulation
| Role | How Triglycerides Help |
|---|---|
| Prevent heat loss | Adipose tissue under the skin reduces heat exchange |
| Maintain body temperature | Essential in cold habitats for thermal balance |
| Support survival in cold | Found in whales, seals, polar bears as blubber |
🧠 Summary Box – Role of Triglycerides in the Body
| Function | Triglyceride Property Involved |
|---|---|
| Long-term energy storage | High energy density, compact, hydrophobic |
| Thermal insulation | Fat layers prevent heat loss |
| Protection of organs | Cushions internal structures |
| Reserve material | Mobilized during fasting or high demand |
⚡ Triglycerides are multifunctional lipids – storing energy, shielding organs, and insulating life from the cold.
B1.1.12 – Formation of Phospholipid Bilayers as a Consequence of Hydrophobic and Hydrophilic Regions
🧬 What Are Phospholipids?
Phospholipids are the essential building blocks of cell membranes. Each phospholipid molecule contains:
– 1 glycerol backbone
– 2 hydrophobic fatty acid tails
– 1 hydrophilic phosphate group
This combination makes phospholipids amphipathic, with both water-attracting and water-repelling regions.
This dual nature is key to forming dynamic membranes.
🌊 Amphipathic Nature of Phospholipids
| Region | Nature | Orientation in Water |
|---|---|---|
| Phosphate head | Hydrophilic | Faces outward towards water |
| Fatty acid tails | Hydrophobic | Faces inward, away from water |
🧱 Formation of the Phospholipid Bilayer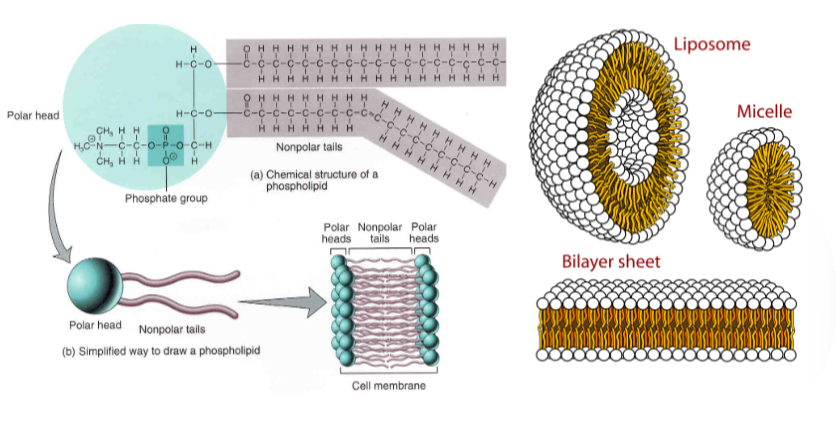
In aqueous environments, phospholipids spontaneously arrange into a bilayer:
- Hydrophilic heads face the cytoplasm and extracellular fluid
- Hydrophobic tails align inward, away from water
This self-assembly:
- Forms the cell’s basic membrane structure
- Creates a selectively permeable barrier
🔍 Why the Bilayer Is So Important
| Feature | Biological Function |
|---|---|
| Amphipathic nature | Drives spontaneous bilayer formation in water |
| Fluidity of the membrane | Allows embedded proteins and lipids to move |
| Semi-permeability | Regulates transport of substances in/out of cell |
| Self-healing | Can repair minor damage naturally |
🧠 Summary Box – Amphipathic Phospholipids & Membranes
| Component | Hydrophilic or Hydrophobic | Role in Bilayer |
|---|---|---|
| Phosphate head | Hydrophilic | Faces water (cytoplasm & ECF) |
| Fatty acid tails | Hydrophobic | Hidden from water; forms inner barrier |
| Whole phospholipid | Amphipathic | Essential for membrane formation |
🌐 Without phospholipids, cells couldn’t create membranes – and without membranes, life as we know it couldn’t exist.
B1.1.13 – Ability of Non-Polar Steroids to Pass Through the Phospholipid Bilayer
🧪 What Are Steroids?
Steroids are a class of lipid-based molecules built from a characteristic four-ring hydrocarbon structure.
They are non-polar, making them insoluble in water but soluble in lipid environments such as cell membranes.
Common biological examples include:
- Oestradiol – an estrogen hormone
- Testosterone – an androgen hormone
💡 Structure of Steroids
| Feature | Description |
|---|---|
| Core structure | 4 fused carbon rings (3 hexagons + 1 pentagon) |
| Functional groups | Small side chains or hydroxyl groups |
| Polarity | Mostly non-polar (hydrophobic) |
🌐 Crossing the Phospholipid Bilayer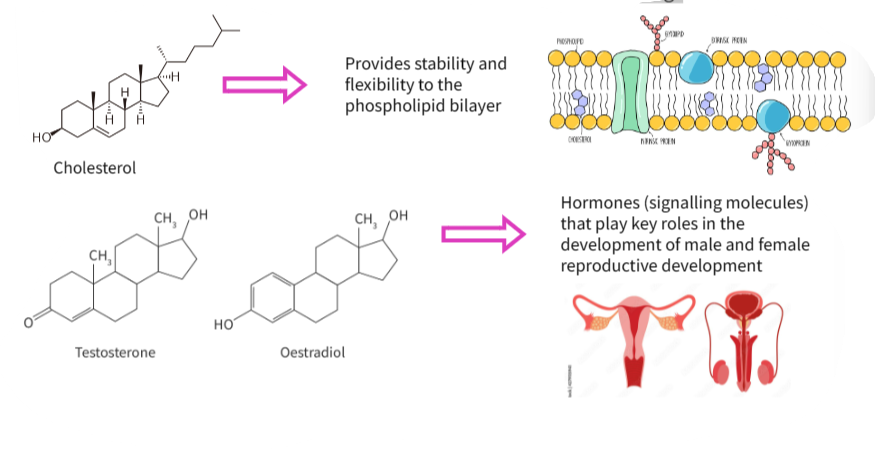
Phospholipid bilayers form the semi-permeable barrier around cells.
Because steroids are non-polar, they can dissolve in the hydrophobic core of the membrane.
This allows them to:
- Pass directly through the phospholipid bilayer
- Enter target cells without the need for transport proteins
🧬 Examples of Steroid Hormones
| Hormone | Type | Produced By | Function |
|---|---|---|---|
| Oestradiol | Estrogen | Ovaries | Regulates female reproductive cycle and secondary sexual characteristics |
| Testosterone | Androgen | Testes, adrenal glands | Regulates male reproductive development and muscle growth |
🔍 How Steroid Hormones Work
– Steroid hormones diffuse directly through the phospholipid bilayer
– Inside the cell, they bind to specific intracellular receptors (often in the nucleus)
– This complex regulates gene expression by turning genes on or off
– Triggering the synthesis of specific proteins
Unlike peptide hormones, steroid hormones act inside the cell, not on the membrane surface.
🧠 Summary Box – Steroids & Membrane Permeability
| Steroid | Polarity | Can Cross Membrane? | Why? |
|---|---|---|---|
| Oestradiol | Non-polar | Yes | Lipid-soluble → diffuses through membrane |
| Testosterone | Non-polar | Yes | No charge → dissolves in membrane lipids |
💊 Steroids bypass surface receptors by slipping right into cells — a clever shortcut used in hormonal signaling.