IB DP Biology Enzymes and metabolism Study Notes - New Syllabus
IB DP Biology Enzymes and metabolism Study Notes
IB DP Biology Enzymes and metabolism Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on IB Biology syllabus with guiding questions of
- In what ways do enzymes interact with other molecules?
- What are the interdependent components of metabolism?
Standard level and higher level: 3 hours
Additional higher level: 2 hours
C1.1.1 – Enzymes as Catalysts
⚡ What is a Catalyst?
- A catalyst is a substance that speeds up a chemical reaction without being used up.
- Only a small amount is needed to make a big difference.
Inorganic Catalyst Example:
Platinum in car exhaust systems helps convert toxic gases into harmless ones.
🧬 What Are Enzymes?
- Enzymes are biological catalysts made by living cells.
- They increase the rate of biochemical reactions that would otherwise be too slow to sustain life.
⚙️ How Enzymes Work
They convert substrates into products in a specific reaction:
Substrate + Enzyme → Product
Enzymes are highly specific each one usually works on one type of reaction.
🚀 Why Are Enzymes Important?
- Speed: Enzymes can accelerate reactions by a factor of 10⁸ to 10²⁶!
- That’s up to 100,000,000,000,000,000,000,000,000 times faster
- Without enzymes, reactions like respiration, digestion, and cell division would be too slow.
- Life couldn’t function properly.
🔥 Activation Energy and Enzyme Action
Activation Energy: Energy needed to start a chemical reaction by breaking bonds in substrates.
Enzymes lower this energy barrier → Reactions start more easily.
With and Without Enzymes:
| Condition | Activation Energy | Reaction Speed |
|---|---|---|
| Without enzyme | High | Slow |
| With enzyme | Lower | Fast |
♻️ Reusable Catalysts
Enzymes are not changed by the reaction.
They can be used over and over again, often millions of times.
– Enzymes are biological catalysts that speed up cellular reactions.
– They work by lowering activation energy, making reactions happen faster.
– Enzymes are specific, reusable, and essential for life processes like respiration and digestion.
– Without enzymes, life would be too slow to survive.
C1.1.2 – Role of Enzymes in Metabolism
💡 What is Metabolism?
Metabolism is the sum of all chemical reactions in a living organism.
It includes:
- Catabolic reactions – breaking down molecules (e.g. respiration).
- Anabolic reactions – building molecules (e.g. protein synthesis).
⚙️ How Enzymes Are Involved
Metabolism involves thousands of different reactions – all controlled by enzymes.
Each reaction is catalysed by a specific enzyme, due to the enzyme’s active site only fitting certain substrates.
This enzyme specificity means:
- Different enzymes are needed for each step in a metabolic pathway.
- The body has many different enzymes, each responsible for a unique reaction.
🧪 Why Enzyme Control Is Important
Enzymes regulate the speed and direction of reactions.
By controlling enzyme activity, cells can:
- Speed up or slow down specific parts of metabolism.
- Switch pathways on or off depending on conditions (e.g. during exercise or fasting).
Example:
The enzyme hexokinase starts glycolysis by phosphorylating glucose.
If glucose is low, the enzyme’s activity is reduced, slowing down the pathway.
🔁 Metabolic Pathways Are Interconnected
Enzyme-controlled reactions form chains or cycles (e.g. the Krebs cycle).
A product of one reaction becomes the substrate for the next.
This network of reactions is highly regulated to:
- Maintain homeostasis
- Respond to internal and external changes
– Metabolism = all the chemical reactions in cells (catabolic + anabolic).
– Each metabolic reaction is controlled by a specific enzyme.
– Enzyme activity allows cells to regulate metabolism efficiently.
– Metabolic control is essential for growth, energy balance, and adaptation.
C1.1.3 – Anabolic and Catabolic Reactions
🔁 What Is the Difference?
Metabolism includes two main types of reactions:
| Type of Reaction | What It Does | Energy | Examples |
|---|---|---|---|
| Anabolism | Builds larger molecules from smaller ones | Requires energy (endergonic) | Protein synthesis, glycogen formation, photosynthesis |
| Catabolism | Breaks down large molecules into smaller ones | Releases energy (exergonic) | Digestion, respiration, hydrolysis of macromolecules |
Anabolic Reactions (Building Up)
Condensation reactions – water is removed to join monomers.
Used to make macromolecules needed for growth and repair.
📌 Examples of Anabolic Reactions:
- Protein synthesis – joins amino acids to make polypeptides.
- Glycogen formation – joins glucose units for energy storage in animals.
- Photosynthesis – uses CO₂ and H₂O to build glucose (a carbohydrate).
General form:
Monomers + Energy → Macromolecule + Water
Catabolic Reactions (Breaking Down)
Hydrolysis reactions – water is added to break bonds.
Provides raw materials and energy for cells.
📌 Examples of Catabolic Reactions:
- Digestion – breaks down proteins, carbs, fats into monomers (e.g. amino acids, sugars, fatty acids).
- Respiration – glucose is broken down to release ATP (cell energy).
General form:
Macromolecule + Water → Monomers + Energy
⚖️ Anabolism vs. Catabolism – Quick Comparison
| Feature | Anabolism | Catabolism |
|---|---|---|
| Direction | Builds molecules | Breaks molecules |
| Energy flow | Requires energy (ATP) | Releases energy |
| Reaction type | Condensation | Hydrolysis |
| Examples | Photosynthesis, protein synthesis | Digestion, respiration |
– Anabolic reactions build complex molecules and need energy.
– Catabolic reactions break molecules down and release energy.
– Both are enzyme-controlled and vital to maintaining life and energy balance.
– They are interdependent—catabolism provides energy for anabolism!
C1.1.4 – Enzymes as Globular Proteins with an Active Site for Catalysis
🧪 What Are Enzymes Made Of?
- Enzymes are globular proteins – compact, water-soluble proteins with a specific 3D shape.
- Their shape is essential for their function as catalysts.
🎯 The Active Site: Where the Action Happens
The active site is the region on the enzyme where the substrate binds.
Made of only a few amino acids, but…
It is perfectly shaped for the substrate – like a lock and key!
🔄 Shape Is Everything!
The 3D structure of the enzyme (its tertiary structure) is formed by interactions between amino acids:
- Hydrogen bonds
- Ionic bonds
- Disulfide bridges
- Hydrophobic interactions
These internal interactions determine the precise shape and chemical properties of the active site.
So, even though the active site is small, it depends on the whole protein’s structure to exist and function properly.
🧬 Why It Matters for Catalysis
- The active site binds the substrate temporarily and lowers the activation energy.
- This allows the chemical reaction to proceed faster without the enzyme being used up.
🔗 Enzyme + Substrate → Enzyme–Substrate Complex → Product + Enzyme
– Enzymes are globular proteins with a precise 3D shape.
– The active site is formed by only a few amino acids but depends on the whole enzyme’s structure.
– Enzymes catalyze reactions by forming enzyme–substrate complexes and lowering activation energy.
– Structure = function – even small changes to the protein can affect enzyme activity!
C1.1.5 – Interactions Between Substrate and Active Site: Induced-Fit Binding
🧠 What Is Induced Fit?
- The induced-fit model is an updated explanation of how enzymes and substrates interact.
- Unlike the lock-and-key model (which suggests a perfect fit), induced fit shows that both enzyme and substrate slightly change shape when they bind.
🔄 How Induced Fit Works
- Substrate approaches the enzyme’s active site.
- The enzyme’s active site molds itself around the substrate — like a hand fitting a glove.
- This flexible binding ensures a tighter and more specific fit.
- It puts strain on substrate bonds, making them easier to break or rearrange during the reaction.
🔬 Why Is This Important?
Induced fit improves:
- Specificity – the enzyme fits only the right substrate.
- Efficiency – helps lower activation energy more effectively.
It explains how some enzymes can act on similar-looking substrates, but not the wrong ones.
📊 Lock-and-Key vs Induced Fit
| Model | Key Idea | Flexibility? | Realistic? |
|---|---|---|---|
| Lock and Key | Substrate fits perfectly into a fixed active site | No | Less |
| Induced Fit | Active site changes shape to fit substrate | Yes | More |
– In the induced-fit model, both enzyme and substrate change shape slightly when binding.
– This improves fit, specificity, and catalytic efficiency.
– Induced fit helps explain how enzymes are flexible yet highly selective.
C1.1.6 – Role of Molecular Motion and Substrate – Active Site Collisions in Enzyme Catalysis
🔄 Why Movement Matters in Enzyme Reactions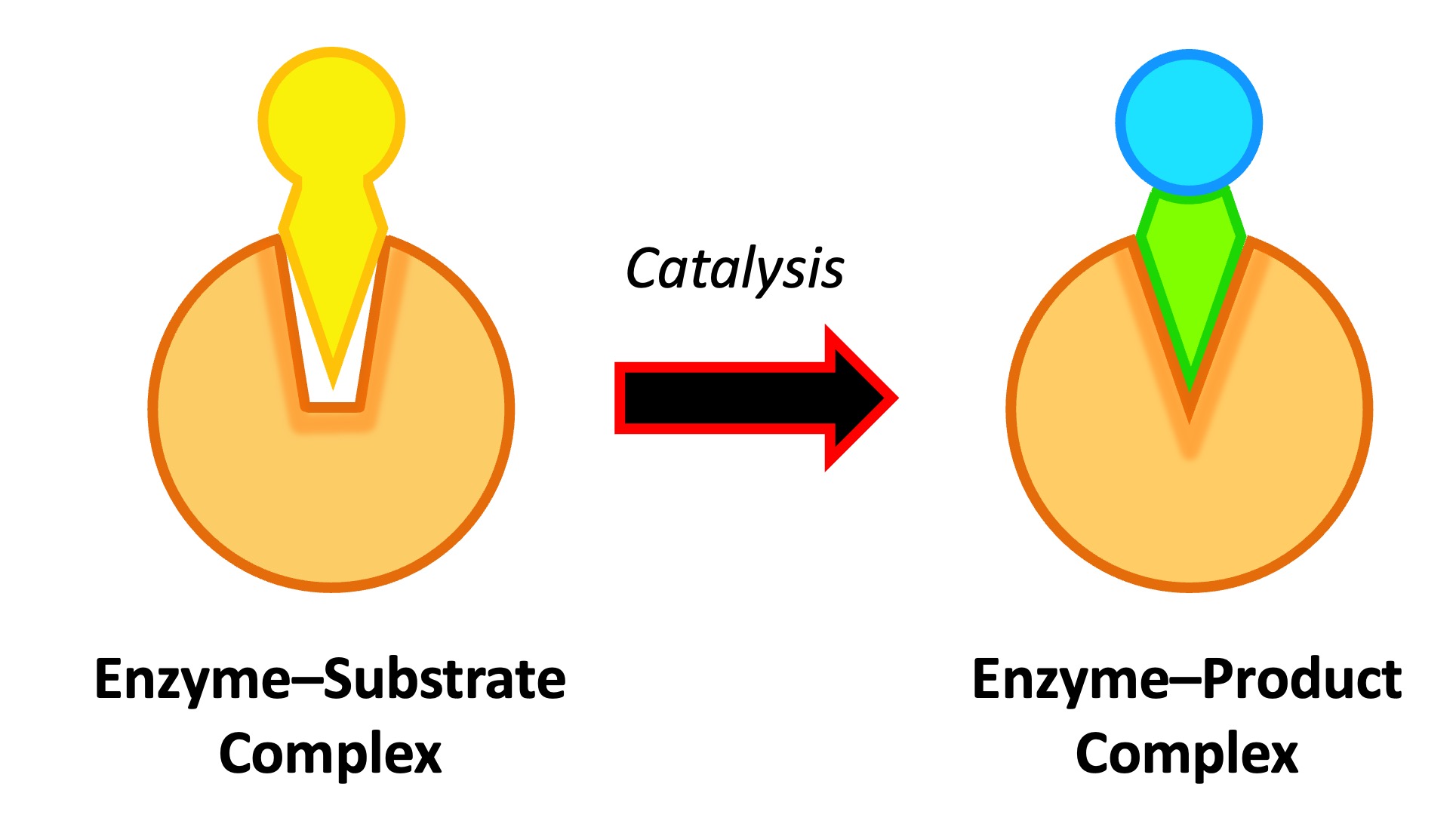
For an enzyme to catalyze a reaction, the substrate molecule must collide with the enzyme’s active site. This happens through molecular motion – the random movement of particles in liquids due to kinetic energy.
🧪 How Molecular Motion Works in Catalysis
- Enzymes and substrates are constantly moving in a fluid environment.
- A successful collision between the active site and substrate is needed to form the enzyme–substrate complex.
- Collisions must occur in the right orientation and with enough energy.
🧠 Key Factors Influencing Collisions
| Factor | Effect |
|---|---|
| Temperature | Increases kinetic energy → more frequent collisions |
| Concentration | Higher enzyme/substrate concentrations → more chances of collision |
| Molecular Size | Smaller molecules move faster → collide more often |
🧷 What If Movement Is Limited?
Sometimes, movement is restricted – but reactions still happen:
- Immobilized Substrates: Large molecules like DNA may be anchored in place. Enzymes must move to them.
- Immobilized Enzymes: Some enzymes are fixed to membranes (e.g., in mitochondria or the small intestine). The substrate must move to reach the enzyme’s active site.
🧪 Example Situations
| Condition | Example | Who Moves? |
|---|---|---|
| Free enzyme & substrate | Digestion in cytoplasm | Both |
| Large substrate | DNA replication enzymes | Enzyme moves |
| Membrane-bound enzyme | Disaccharidase in intestinal lining | Substrate moves |
– Enzyme catalysis relies on movement and collisions between substrate and active site.
– Successful collisions must have the right orientation and enough energy.
– In some cases, either the enzyme or substrate is immobilized, but the reaction still proceeds thanks to the motion of the other.
– Enzyme reactions are all about chance encounters-helped by molecular motion!
C1.1.7 – Relationships Between the Structure of the Active Site, Enzyme-Substrate Specificity and Denaturation
1. Enzyme-Substrate Specificity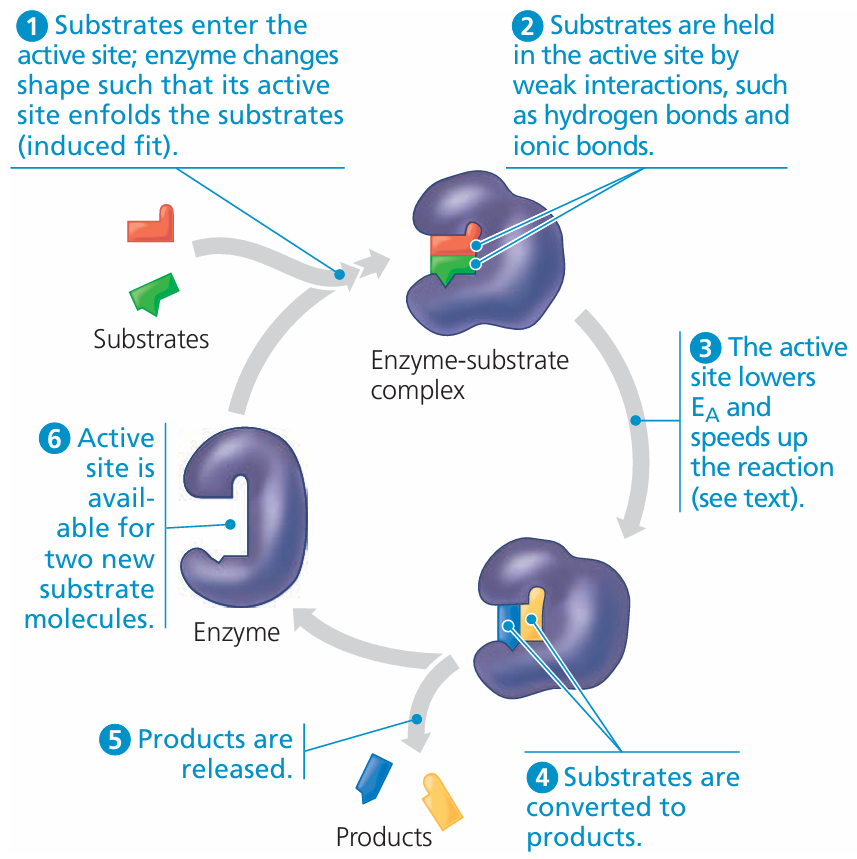
Enzymes are highly specific – they only work with certain substrates because of the unique shape and chemistry of their active site.
🔑 The Lock-and-Key Model:
- The enzyme’s active site fits the substrate like a key fits a lock.
- Only the correct substrate can bind.
🧤 Induced Fit Model:
- The active site slightly changes shape to better fit the substrate.
- This enhances binding and catalysis.
🎯 Specificity Types:
| Type | Description | Example |
|---|---|---|
| Absolute | Binds only one specific substrate | Glucokinase (glucose only) |
| Group specificity | Binds similar types of molecules | Hexokinase (all hexoses) |
| Broad | Binds a range of substrates with shared features | Proteases (many polypeptides) |
2. Structure of the Active Site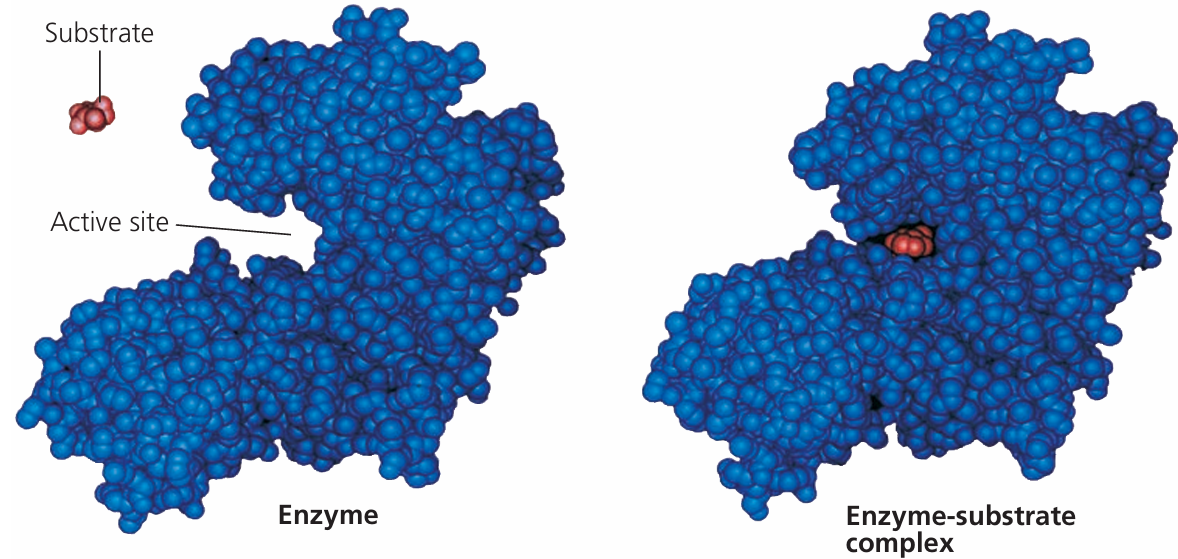
- The active site is made from a few amino acids in the enzyme’s 3D structure.
- These amino acids form hydrogen bonds, ionic interactions, and hydrophobic pockets to attract and bind substrates.
- The overall shape and chemical environment of the active site determines which substrate it can bind.
3. Denaturation Destroys Function
When an enzyme loses its shape, it’s called denaturation.
Causes of Denaturation:
- High heat
- Extreme pH
- Alcohol or heavy metals
Effects of Denaturation:
- Active site changes shape.
- Substrate can’t bind properly.
- Enzyme loses activity, often permanently.
🧠 Visual Summary
| Feature | Normal Enzyme | Denatured Enzyme |
|---|---|---|
| Active site shape | Specific and functional | Altered or destroyed |
| Substrate binding | Successful | Unsuccessful |
| Reaction rate | High | Low or none |
– Enzyme specificity depends on the unique shape and chemistry of the active site.
– The active site’s shape is maintained by the enzyme’s 3D protein structure.
– Denaturation disrupts this structure and stops the enzyme from working.
– Maintaining proper temperature and pH is essential for enzyme function!
C1.1.8 – Effects of Temperature, pH, and Substrate Concentration on Enzyme Activity
⚙️ Enzyme Activity & Reaction Rates: The Basics
- The rate of an enzyme-catalyzed reaction depends on:
- How frequently enzyme and substrate molecules collide (collision theory)
- Whether the enzyme’s active site remains functional (not denatured)
1. Effect of Temperature
| Temperature | Effect |
|---|---|
| Increasing temp | Molecules move faster, increasing collision frequency → Faster reaction |
| Optimum temp | Temperature where the enzyme works most efficiently |
| Too hot | Enzyme structure is denatured – active site changes shape → Reaction stops |
Graph Shape: Rises steeply, peaks, then drops sharply (due to denaturation)
2. Effect of pH
- Each enzyme has an optimum pH (often neutral for human enzymes)
- Too acidic or too basic? ➜ Hydrogen and ionic bonds in enzyme disrupted
- This causes the active site to change → denaturation
Graph Shape: Bell-shaped curve — peak at optimum pH
3. Effect of Substrate Concentration
| Substrate Level | Effect |
|---|---|
| Low substrate | Fewer collisions → slower reaction |
| Increasing substrate | More frequent collisions → faster reaction |
| Enzyme saturation | All active sites are occupied — reaction rate levels off |
Graph Shape: Rapid increase, then plateaus
📊 Interpreting Graphs – Application Skill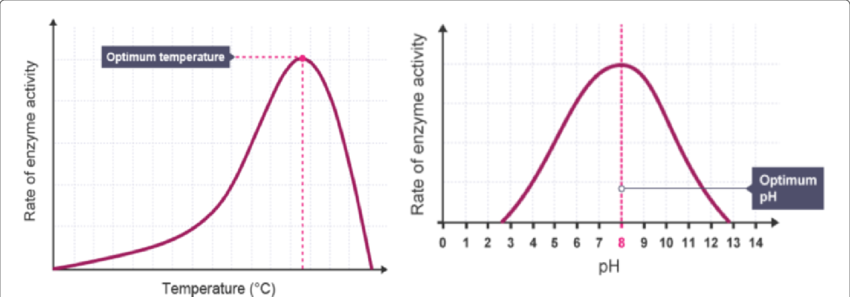
- Identify independent (x-axis) and dependent (y-axis) variables
- Describe relationships:
- “As temperature increases, enzyme activity rises until an optimum is reached, after which it decreases.”
- “Substrate concentration increases the reaction rate until a plateau is reached.”
- Recognize models:
- Sketch graphs are simplified models based on biological experiments.
- These models can be evaluated by comparing them to real data from enzyme experiments.
– Temperature, pH, and substrate concentration affect enzyme activity by influencing molecular motion and enzyme structure.
– Collision theory explains how more movement (temperature or substrate) leads to faster reactions.
– Denaturation (from extreme temp or pH) destroys the active site and stops the reaction.
– Graphs are models that help predict enzyme behavior and can be tested in real experiments.
C1.1.9 – Measurements in Enzyme-Catalysed Reactions
🎯 Purpose of Measuring Enzyme Activity
- To understand how fast an enzyme works
- To see how different factors (like temperature or pH) affect the reaction rate
How Can Enzyme Activity Be Measured?
- 📉 Decrease in substrate concentration
- 📈 Increase in product concentration
Examples:
| Enzyme | Reaction Measured | Measurement Method |
|---|---|---|
| Catalase | Hydrogen peroxide → Water + Oxygen | Measure volume of O₂ gas released |
| Amylase | Starch → Maltose | Use iodine test: less blue-black colour = more starch broken down |
| Lipase | Lipid → Fatty acids | Use pH indicator: solution becomes more acidic |
🧪 Using Experiments or Secondary Data
Experimental Skills:
- Set up controlled experiments
- Record results at regular time intervals
- Repeat and calculate mean rates
Working with Secondary Data:
- Use graphs or tables provided from published data
- Determine:
- Initial rate (steepest part of graph)
- Average rate (total product ÷ time)
- Compare results from different conditions
🔢 How to Calculate Rate of Reaction
Use the formula:
Rate = Change in amount (product or substrate) ÷ Time taken
Example:
If 5 cm³ of oxygen is produced in 20 seconds:
Rate = 5 ÷ 20 = 0.25 cm³/s
📈 Interpreting Graphs
- Linear section = steady enzyme activity
- Plateau = substrate used up / product buildup
- Steep initial slope = fastest reaction rate
🧠 Skill Tip: Identify the initial rate from the tangent at time = 0 on a graph.
– Enzyme activity can be measured by tracking substrate loss or product formation.
– Use the formula: Rate = Change ÷ Time
– Experimentation and secondary data help analyze how enzymes work under different conditions.
– Graphs and calculations are essential tools to interpret enzyme-catalysed reactions.
C1.1.10 – Effect of Enzymes on Activation Energy
💥 What Is Activation Energy?
- Activation energy (Ea) is the minimum amount of energy needed to start a chemical reaction.
- It’s the energy required to break bonds in the substrate so the reaction can begin.
⚙️ How Enzymes Help
- Enzymes lower the activation energy, so reactions happen faster and more easily.
Key points:
- Enzymes don’t add energy they reduce the energy barrier.
- This means more molecules can react with the available energy.
- The products still release energy when new bonds are formed.
📉 Graph: With vs Without Enzyme
Here’s what a typical energy profile graph shows:
| Feature | Without Enzyme | With Enzyme |
|---|---|---|
| Activation Energy | High peak | Lower peak |
| Reactants → Products | Same energy difference (ΔE) | Same ΔE |
| What changes? | Only the size of the energy “hill” | Enzyme lowers the hill |
📊 Graph Skills: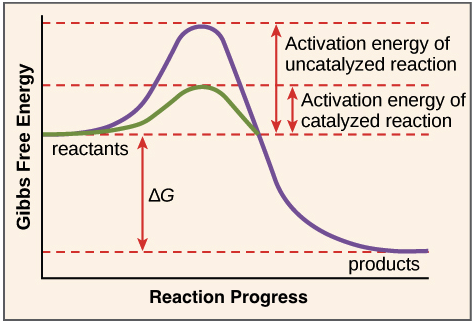
- Identify activation energy as the height from reactants to peak.
- Enzyme-catalysed reactions have a lower peak = lower Ea.
🧪 Energy in Enzyme-Catalysed Reactions
- Bond breaking (in substrate) requires energy = endothermic step.
- Bond forming (in product) releases energy = exothermic step.
- Enzymes speed this up by stabilizing the transition state.
Application of Skills
- Interpret reaction pathway diagrams
- Compare Ea with and without enzyme
- Understand that enzymes make reactions more efficient, not more powerful
– Activation energy is the energy needed to start a reaction by breaking bonds.
– Enzymes lower activation energy, making reactions faster and easier.
– Graphs show a lower energy peak when an enzyme is used.
– The total energy change (ΔE) of the reaction remains the same with or without an enzyme.
Additional Higher Level
C1.1.11 – Intracellular and Extracellular Enzyme-Catalysed Reactions
🔍 What Are Enzyme-Catalysed Reactions?
- Enzymes speed up biochemical reactions by lowering activation energy.
- These reactions can happen:
- Inside cells = intracellular
- Outside cells = extracellular
🏠 Intracellular Enzyme Reactions (Inside the Cell)
These are enzyme-controlled reactions that happen within the cell’s cytoplasm or organelles.
Key examples:
| Process | Location | Enzymes Involved | Function |
|---|---|---|---|
| Glycolysis | Cytoplasm | Kinases, isomerases, dehydrogenases | Breaks glucose into pyruvate (first stage of respiration) |
| Krebs Cycle | Mitochondrial matrix | Dehydrogenases, decarboxylases | Produces NADH, FADH₂, ATP and CO₂ in aerobic respiration |
These reactions are essential for producing energy and intermediates needed by the cell.
🌐 Extracellular Enzyme Reactions (Outside the Cell)
These happen outside the cell usually in the digestive system.
Example: Chemical Digestion in the Gut
| Enzyme | Site of Action | Substrate | Product |
|---|---|---|---|
| Amylase | Mouth & small intestine | Starch | Maltose |
| Protease (e.g. pepsin, trypsin) | Stomach, small intestine | Proteins | Amino acids |
| Lipase | Small intestine | Lipids | Fatty acids + glycerol |
These enzymes are secreted by cells (e.g. by salivary glands, pancreas) but act outside the cell to help digestion.
⚖️ Comparison Table
| Feature | Intracellular | Extracellular |
|---|---|---|
| Location | Inside cell (cytoplasm, organelles) | Outside cell (e.g. digestive system) |
| Role | Energy production, metabolism | Breakdown of food molecules |
| Examples | Glycolysis, Krebs cycle | Digestion by amylase, protease, lipase |
| Reuse of Enzymes | Often reused within the cell | Often secreted and replaced |
– Intracellular reactions happen inside cells and are vital for metabolism (e.g. glycolysis, Krebs cycle).
– Extracellular reactions occur outside cells, mainly in digestion (e.g. enzymes in the gut).
– Both types are catalysed by specific enzymes to ensure speed and efficiency of biological processes.
C1.1.12 – Generation of Heat Energy by the Reactions of Metabolism
💡 Why Is Heat Produced in Metabolism?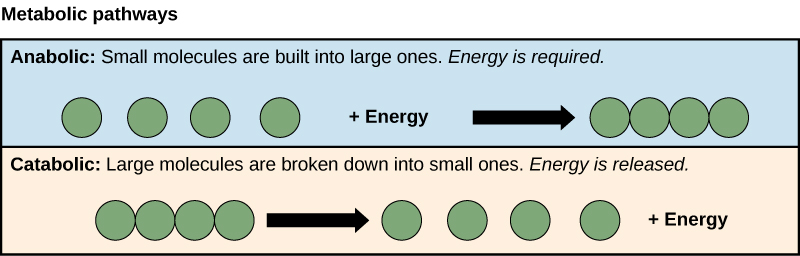
Metabolism = the sum of all chemical reactions in the body. These reactions include:
- Catabolic reactions (breaking down molecules)
- Anabolic reactions (building new molecules)
Every time energy is transferred during these reactions, some of it is lost as heat.
⚙️ Energy Transfer ≠ 100% Efficient
- Biological reactions are never 100% efficient.
- Some energy from glucose, fats, and proteins is transferred to ATP.
- The rest is lost as heat – this is a natural consequence of energy transfer.
Example: In cellular respiration, only ~40% of the energy in glucose is captured in ATP. The remaining ~60% is released as heat.
Why Is Heat Important?
🌡️ Thermoregulation in Endotherms (Warm-blooded Animals)
- Animals like mammals and birds rely on this metabolic heat to:
- Maintain a constant internal body temperature
- Function in cold environments
- Support enzymatic reactions, which work best at optimum temperature (around 37°C in humans)
⚠️ Without this heat:
- Enzyme activity would slow down
- Body processes would become inefficient or stop
- Survival in colder habitats would be difficult
🔬 Real-World Examples
| Animal | How It Uses Metabolic Heat |
|---|---|
| Human | Keeps body at 37°C through respiration heat |
| Birds | Maintain high body temperatures for flight muscles |
| Polar bear | Combines thick fur and metabolic heat to survive arctic cold |
♻️ Heat Is Always a Byproduct
Even in aerobic respiration, where ATP is efficiently produced, heat is always generated due to:
- Friction between molecules
- Inefficiencies in biochemical pathways
- Energy losses during ATP synthesis
– Heat production is an inevitable byproduct of metabolism due to energy transfer inefficiencies.
– Mammals and birds depend on metabolic heat to maintain a constant body temperature.
– This heat helps support optimal enzyme activity and survival in variable environments.
C1.1.13 – Cyclical and Linear Pathways in Metabolism
🧬 What Is a Metabolic Pathway?
A metabolic pathway is a series of enzyme-catalysed chemical reactions in a cell. These can either be:
- Linear pathways: reactions proceed in one direction.
- Cyclical pathways: the end product is also the starting compound, so the cycle repeats.
Linear Pathways 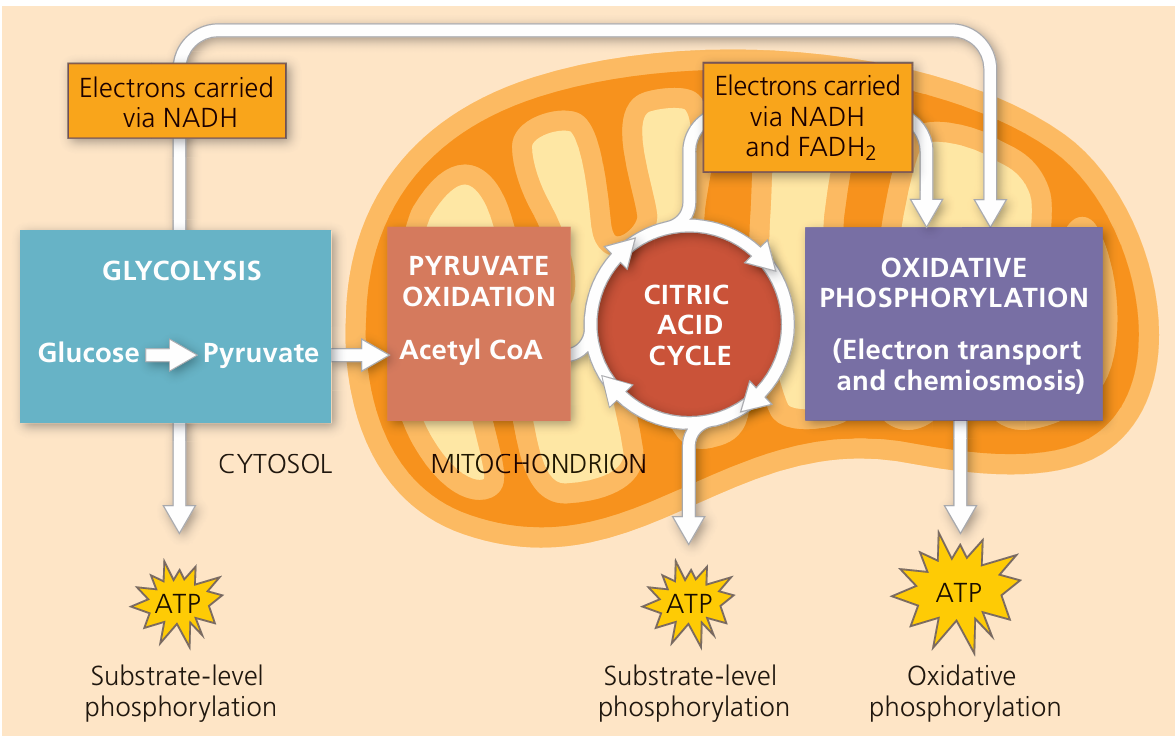
➡️ One-way, Step-by-Step Reactions
Substrates are converted through a sequence of intermediates into a final product.
The pathway does not loop back.
Example: Glycolysis
- Occurs in the cytoplasm.
- Breaks glucose (6-carbon sugar) into two pyruvate molecules (3-carbon).
- Net gain: 2 ATP and 2 NADH.
- It’s a linear pathway — no steps repeat or loop.
Cyclical Pathways
🔄 The Last Step Regenerates the First Reactant
Products of the last step feed back into the first step.
Continuous cycle as long as substrates and enzymes are present.
📘 Examples:
| Cycle | Where It Happens | Purpose |
|---|---|---|
| Krebs Cycle (Citric Acid Cycle) | Mitochondrial matrix | Releases energy by oxidizing acetyl-CoA; produces ATP, NADH, FADH₂, CO₂ |
| Calvin Cycle | Chloroplast stroma | Uses CO₂, ATP, NADPH to build glucose in photosynthesis |
🔬 Comparison Table
| Feature | Linear Pathway | Cyclic Pathway |
|---|---|---|
| Direction | One-way | Repeats in a loop |
| Example | Glycolysis | Krebs cycle, Calvin cycle |
| Main Function | Energy release (e.g. ATP) | Energy transformation, carbon fixation |
| Occurs in | Cytoplasm (e.g. glycolysis) | Mitochondria or chloroplast |
– Linear pathways have a start and end (e.g. glycolysis).
– Cyclic pathways regenerate the starting molecule and repeat (e.g. Krebs and Calvin cycles).
– Both types are crucial for energy production, carbon fixation, and biosynthesis in cells.
C1.1.14 – Allosteric Sites and Non-Competitive Inhibition
🧬 What is an Allosteric Site?
An allosteric site is a region on an enzyme away from the active site.
- Specific molecules (called allosteric effectors) can bind here.
- Binding causes a conformational change (change in 3D shape) of the enzyme.
- This change can alter the shape of the active site, reducing or blocking enzyme activity.
🚫 Non-Competitive Inhibition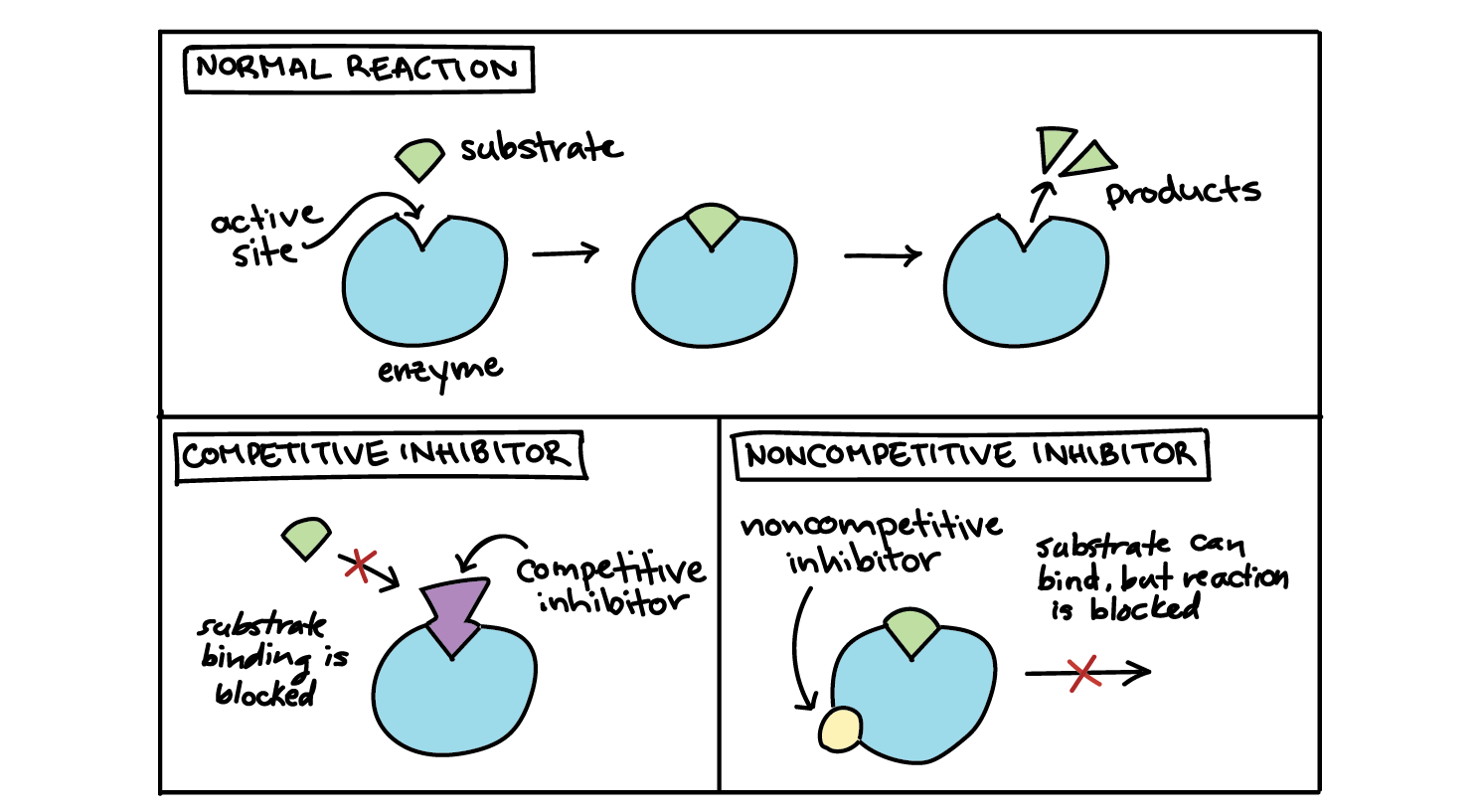
A non-competitive inhibitor binds to the allosteric site, not the active site.
- Binding is usually reversible.
- It causes a shape change in the enzyme that prevents the substrate from binding properly at the active site.
- Even if substrate concentration increases, the inhibitor’s effect doesn’t go away.
🧩 How It Works: Step-by-Step
- Substrate wants to bind to the active site.
- A non-competitive inhibitor binds to the allosteric site.
- This causes a structural change in the enzyme.
- The active site is distorted, so it can no longer catalyze the reaction.
🔬 Key Features
| Feature | Description |
|---|---|
| Binding site | Allosteric site (not active site) |
| Effect on enzyme | Changes shape of active site |
| Reversibility | Binding is reversible |
| Overcome by substrate? | No |
| Effect on reaction rate | Decreases Vmax (maximum rate of reaction) |
Real Example
Cyanide inhibits cytochrome c oxidase (an enzyme in respiration) by binding to a non-active site, stopping ATP production a deadly non-competitive inhibitor.
– Allosteric sites are special sites where specific molecules bind.
– Non-competitive inhibitors use these sites to stop catalysis by changing enzyme shape.
– This inhibition is usually reversible, but cannot be overcome by adding more substrate.
– It’s a powerful way cells regulate enzymes.
C1.1.15 – Competitive Inhibition: A Battle for the Active Site
🎯 What is Competitive Inhibition?
In competitive inhibition, the inhibitor is a molecule similar in shape to the enzyme’s substrate.
- It binds reversibly to the active site, blocking the substrate from attaching.
- Both the substrate and inhibitor compete for the same active site.
How It Works
- The inhibitor resembles the substrate and fits into the enzyme’s active site.
- While the inhibitor is bound, the real substrate cannot enter.
- No product is formed while the inhibitor occupies the site.
- The inhibition is reversible – the inhibitor can detach, allowing the substrate another chance.
Example: Statins
Statins are drugs that competitively inhibit the enzyme HMG-CoA reductase, involved in cholesterol synthesis.
- They reduce cholesterol levels by blocking the substrate from binding to the enzyme.
📈 Effect of Substrate Concentration
| Condition | Outcome |
|---|---|
| Low substrate concentration | Inhibitor is more likely to bind, slowing the reaction |
| High substrate concentration | Substrate outcompetes the inhibitor → reaction rate increases |
⚖️ Competitive vs Non-Competitive Inhibition
| Feature | Competitive Inhibition | Non-Competitive Inhibition |
|---|---|---|
| Binding site | Active site | Allosteric site |
| Reversibility | Yes | Yes |
| Substrate competes? | Yes | No |
| Effect of more substrate | Can overcome inhibition | Cannot overcome |
| Effect on Vmax | Unchanged | Decreases |
| Effect on rate at low [substrate] | Reduced | Reduced |
– Competitive inhibitors bind reversibly to the active site, blocking the substrate.
– Statins are real-world competitive inhibitors used to lower cholesterol.
– Increasing substrate concentration can reverse the inhibition.
– Competitive inhibition does not reduce the enzyme’s maximum potential, only slows it down at low substrate levels.
C1.1.16 – Feedback Inhibition in Metabolic Pathways
⚙️ What Is Feedback Inhibition?

Feedback inhibition is a type of negative feedback used to regulate metabolic pathways.
- The end product of a metabolic pathway acts as an inhibitor to an earlier enzyme in the pathway.
- This prevents overproduction of the end product – like a biological “off switch.”
🧪 How It Works
- A metabolic pathway involves a series of enzyme-controlled steps that convert a starting molecule into a final product.
- When enough of the end product is made, it binds to an enzyme that catalyzes an early step.
- This inhibits the enzyme’s activity – slowing or stopping the entire pathway.
- When the end product is used up, the inhibition lifts, and the pathway resumes.
Example: Isoleucine Biosynthesis
- Isoleucine is an amino acid produced from threonine in a five-step metabolic pathway.
- The first enzyme in this pathway is threonine deaminase.
- When isoleucine builds up, it binds allosterically to threonine deaminase.
- This changes the enzyme’s shape, preventing it from binding to threonine.
- As a result, no more isoleucine is made until the level drops again.
🧩 Why Feedback Inhibition Is Useful
- Prevents waste of resources and energy.
- Maintains homeostasis in the cell.
- Ensures balanced production of essential molecules.
– Feedback inhibition is when the end product of a pathway inhibits an earlier enzyme.
– It’s a form of non-competitive, reversible inhibition via allosteric sites.
– Isoleucine inhibits threonine deaminase when enough has been made.
– This process helps cells avoid overproduction and conserve resources.
C1.1.17 – Mechanism-Based Inhibition (Irreversible Inhibitors)
🔍 What Is Mechanism-Based Inhibition?
Also known as suicide inhibition, this occurs when:
- An inhibitor binds to the active site of an enzyme,
- Then undergoes a chemical reaction that permanently changes the enzyme.
- The result? The enzyme is irreversibly inactivated – it’s no longer functional.
Example: Penicillin and Bacterial Enzymes
- Target: Transpeptidase
- Penicillin targets transpeptidase enzymes in bacteria.
- These enzymes help cross-link peptidoglycan – a key bacterial cell wall component.
How Penicillin Works:
- Penicillin resembles the enzyme’s natural substrate.
- It binds to the active site of transpeptidase.
- A chemical reaction forms a covalent bond with the enzyme.
- This permanently disables the enzyme → No cell wall formation → Bacterial cell bursts.
🧬 Resistance to Penicillin: A Molecular Arms Race
- Some bacteria evolve by altering their transpeptidase enzyme.
- These altered enzymes (called penicillin-binding proteins, PBPs) no longer bind penicillin well.
- Example: MRSA (Methicillin-resistant Staphylococcus aureus) has resistant PBPs.
⚠️ Differences from Reversible Inhibition
| Feature | Reversible Inhibition | Mechanism-Based (Irreversible) Inhibition |
|---|---|---|
| Binding | Weak, non-covalent | Strong, covalent |
| Duration | Temporary | Permanent |
| Enzyme recovery | Possible | Enzyme must be replaced by new synthesis |
– Mechanism-based inhibitors permanently disable enzymes through chemical modification.
– Penicillin inhibits transpeptidase, blocking bacterial cell wall synthesis.
– Some bacteria evolve resistant enzymes (PBPs) that prevent penicillin binding.
– Unlike reversible inhibitors, this process is irreversible and enzyme-destroying.
