C1.2.1 – ATP: The Cell’s Energy Currency
🧪 What is ATP?
ATP stands for Adenosine Triphosphate. It’s a nucleotide, composed of:
- Adenine (a nitrogenous base)
- Ribose (a 5-carbon sugar)
- Three phosphate groups (the key to its energy-storing ability)
Why ATP is Called the “Energy Currency”
Just like money is used to pay for goods, ATP is used to “pay” for energy-requiring processes in cells.

ATP is made in one place (like mitochondria) and used elsewhere (like muscles or during protein synthesis).
💥 How ATP Stores and Releases Energy
The energy is stored in the bonds between phosphate groups – especially the last (terminal) phosphate bond.
When ATP is broken down:
ATP → ADP + Pi + energy
- ADP = Adenosine diphosphate
- Pi = Inorganic phosphate
This reaction is exergonic (releases energy) and occurs quickly ideal for cells that need instant energy.
Properties That Make ATP Ideal
| Property | Why It’s Useful |
|---|---|
| Small and soluble | Easily moves around inside cells |
| Releases energy in small amounts | Prevents overheating or waste of energy |
| Rapid breakdown and reformation | Can be recycled quickly and reused |
| Universal molecule | Used in all types of cells, across all life forms |
| Couples with many reactions | Drives both anabolic and catabolic processes |
🔁 ATP in Action: Uses in Cells
- Muscle contraction
- Active transport (e.g., sodium-potassium pump)
- Protein synthesis
- DNA replication
- Cell signalling and metabolic control
– ATP (adenosine triphosphate) is a nucleotide used by all cells to store and transfer energy.
– It releases energy when the terminal phosphate is removed (forming ADP + Pi).
– Its small, soluble, and recyclable nature makes it perfect as the universal energy currency in biology.
C1.2.2 – Life Processes Powered by ATP
🔋 ATP: Fuel for Cellular Activities
ATP provides energy for many essential processes inside cells. Because it releases energy quickly and in usable amounts, it’s the primary energy source for most life processes.
⚙️ Processes That Require ATP
1. Active Transport Across Membranes
- ATP provides energy for carrier proteins (pumps) that move substances against their concentration gradient.
- Example: Sodium-potassium pump in nerve cells
- Without ATP, substances would only move passively (with the gradient).
2. Anabolic Reactions (Building Macromolecules)
ATP powers condensation reactions that build complex molecules from smaller ones.
- Protein synthesis (from amino acids)
- DNA/RNA synthesis (from nucleotides)
- Glycogen synthesis (from glucose)
3. Movement Within or by Cells
| Type of Movement | ATP Role |
|---|---|
| Chromosome movement | Powers spindle fibers during mitosis and meiosis |
| Cytoplasmic streaming | Helps move organelles through the cytoplasm |
| Muscle contraction | ATP is needed to detach myosin heads from actin |
| Flagella/cilia movement | e.g., sperm movement; ATP powers motor proteins like dynein |
🔁 Summary Table
| Life Process | How ATP Helps |
|---|---|
| Active transport | Moves substances against the gradient |
| Macromolecule synthesis | Provides energy to form chemical bonds |
| Chromosome movement | Drives the mitotic spindle |
| Cell movement (flagella) | Powers motor proteins |
| Organelle transport | Moves vesicles via cytoskeletal tracks |
– ATP is essential for active transport, anabolism, and cell movement.
– These energy-requiring processes would not happen efficiently without ATP.
– ATP ensures that cells remain active, organised, and responsive to their environment.
C1.2.3 – Energy Transfers Between ATP and ADP
💥 ATP: A Rechargeable Energy Source
ATP (adenosine triphosphate) acts like a rechargeable battery for the cell. Its ability to release and store energy lies in the interconversion between ATP and ADP (adenosine diphosphate).
🔽 ATP → ADP + Pi (Hydrolysis)
This reaction releases energy used for cellular processes.
Pi stands for inorganic phosphate (PO₄³⁻).
The reaction:
ATP → ADP + Pi + Energy
⛏️ Process: Hydrolysis
- A water molecule is used to break the bond between the last two phosphate groups.
- Energy is released and immediately used by the cell.
🔼 ADP + Pi → ATP (Synthesis)
This reaction requires energy input.
The reaction:
ADP + Pi + Energy → ATP
🔧 Process: Phosphorylation
- Energy (e.g., from respiration or photosynthesis) is used to rejoin the phosphate group to ADP.
- This stores energy in the new ATP molecule.
🔁 ATP Cycle Diagram
| Reaction | Type | Energy? | What Happens |
|---|---|---|---|
| ATP → ADP + Pi | Hydrolysis | Energy released | Used by cell |
| ADP + Pi → ATP | Phosphorylation | Energy required | Energy stored |
– ATP stores energy in its phosphate bonds; breaking a bond releases energy.
– Hydrolysis of ATP powers most cellular activities.
– ATP is continuously recycled from ADP using energy from respiration or photosynthesis.
– Although students don’t need to know the exact amount of energy released, it is enough to power many essential life processes.
C1.2.4 – Cell Respiration as a System for Producing ATP
💡 What Is Cell Respiration?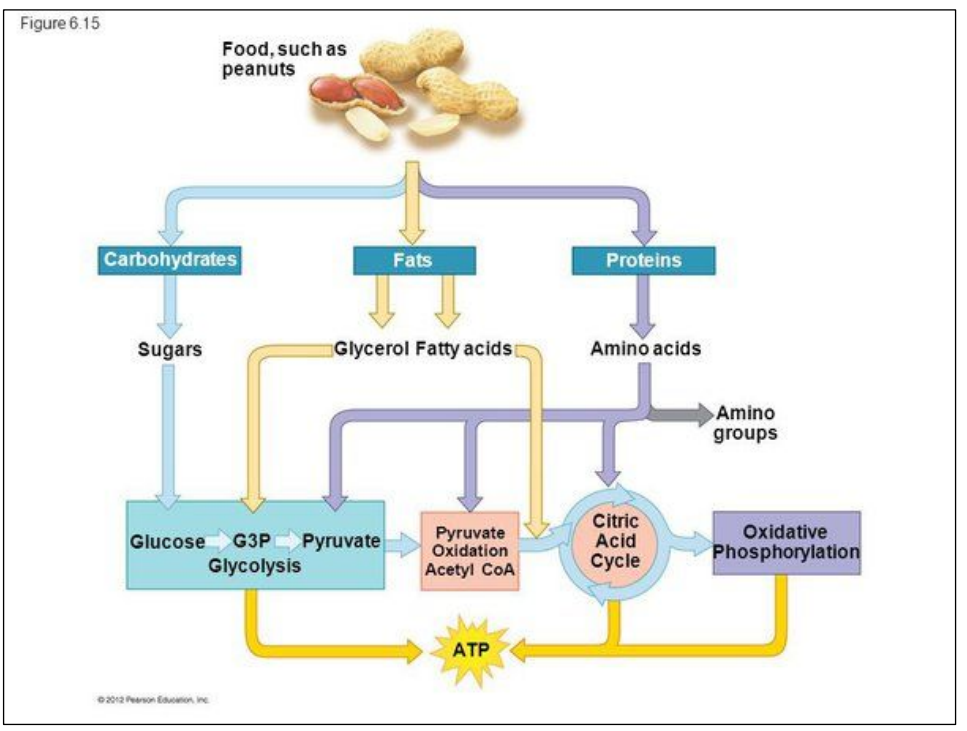
Cell respiration is a metabolic process that breaks down carbon compounds (like glucose and fatty acids) to release energy, which is then used to produce ATP – the cell’s energy currency.
🧪 Main Idea: Energy from Carbon Fuels
- Glucose (a simple sugar) is the main fuel.
- Fatty acids from lipids are also major energy-rich substrates.
- Proteins and other organic compounds can also be used when necessary, though not preferred.
⚙️ Process Overview
- Carbon compounds (e.g., glucose) are broken down in a series of enzyme-controlled steps.
- Energy released from these reactions is used to convert ADP + Pi → ATP.
- Byproducts: Carbon dioxide (CO₂) and water (H₂O).
📊 Key Distinction: Cell Respiration vs. Gas Exchange
| Feature | Cell Respiration | Gas Exchange |
|---|---|---|
| What it is | Chemical reactions that release energy | Physical process of moving gases in/out of cells |
| Purpose | To make ATP | To supply O₂ and remove CO₂ |
| Occurs in | Cytoplasm and mitochondria | Across cell membranes (e.g., lungs, leaves) |
| Uses/produces gases? | Uses O₂ (aerobic) and produces CO₂ | Brings in O₂ and expels CO₂ |
🔋 Why It Matters
Cell respiration powers all life activities by supplying ATP.
Without it, cells would not have the energy to grow, move, divide, or function.
– Cell respiration uses glucose and fatty acids to release energy and make ATP.
– It’s different from gas exchange, which just involves the movement of gases.
– ATP production is the primary goal of cell respiration, enabling cells to perform work.
– Other organic molecules can also be used when needed, showing the flexibility of metabolism.
C1.2.5 – Differences Between Anaerobic and Aerobic Cell Respiration in Humans
⚡ What Is Cell Respiration Again?
It’s how cells release energy from glucose to make ATP. This can happen with or without oxygen, leading to two different types:
🔄 Two Types of Respiration
| Feature | Aerobic Respiration | Anaerobic Respiration |
|---|---|---|
| Oxygen required? | Yes | No |
| Main substrate | Glucose (can also use fatty acids, amino acids) | Glucose only |
| ATP yield | High (~36–38 ATP per glucose) | Low (only 2 ATP per glucose) |
| Waste products | Carbon dioxide (CO₂) + water (H₂O) | Lactic acid (lactate) |
| Where in the cell? | Starts in cytoplasm, finishes in mitochondria | Entirely in cytoplasm |
| Mitochondria needed? | Yes | No |
🧾 Word Equations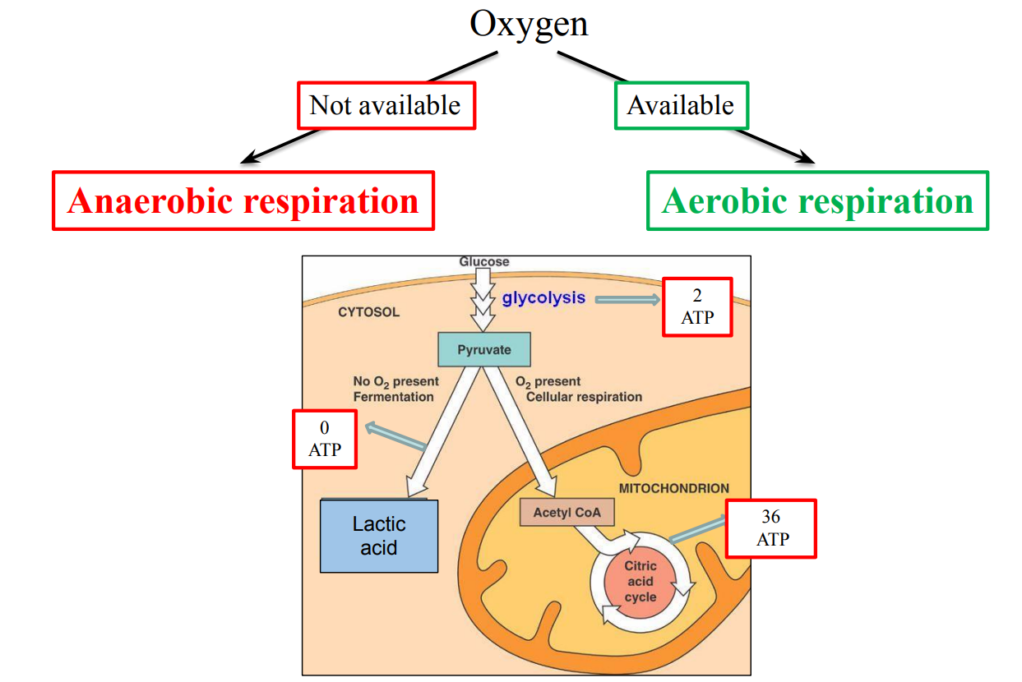
- Aerobic Respiration:
Glucose + oxygen → carbon dioxide + water + energy (ATP) - Anaerobic Respiration (in humans):
Glucose → lactic acid + energy (ATP)
🧬 Location in the Cell
- Aerobic:
Starts in cytoplasm (glycolysis) → Continues in mitochondria (Krebs cycle + electron transport chain) - Anaerobic:
Entirely in the cytoplasm (no mitochondria needed)
📘 Why Both Matter
Aerobic respiration is used most of the time because it’s much more efficient.
Anaerobic respiration kicks in when oxygen is limited, like during intense exercise. It’s faster but produces less ATP and causes muscle fatigue due to lactic acid buildup.
– Aerobic respiration needs oxygen and occurs in the mitochondria, giving high ATP.
– Anaerobic respiration happens in low oxygen conditions in the cytoplasm, producing less ATP and lactic acid.
– Glucose is used in both, but only aerobic respiration can fully break it down into CO₂ and H₂O.
– Word equations help summarize both pathways clearly.
C1.2.6 – Variables Affecting the Rate of Cell Respiration
⚙️ What Is Cell Respiration Rate?
The rate of cell respiration refers to how quickly a cell produces ATP by breaking down glucose (or other organic compounds). It’s often measured by tracking:
- Oxygen consumption
- Carbon dioxide production
- Change in pH
- Heat released
📊 Key Variables That Affect the Rate
| Variable | Effect |
|---|---|
| Temperature | Higher temps increase enzyme activity → faster rate (to a point). Too hot = denaturation. |
| pH | Each enzyme has an optimal pH. Deviations slow down or stop respiration. |
| Glucose concentration | More glucose = more fuel = higher rate (up to a saturation point). |
| Oxygen availability | Needed for aerobic respiration. Limited oxygen shifts cells to slower anaerobic respiration. |
| Enzyme concentration | More enzymes = faster reaction, as long as substrate is available. |
| Cell type or tissue type | Some cells (like muscles) have more mitochondria = higher respiration rates. |
🧪 How to Measure Respiration Rate (Practical Skill)
| Method | What You Measure |
|---|---|
| Respirometer (with seeds or insects) | Volume of oxygen consumed |
| CO₂ probe | Rate of carbon dioxide production |
| pH meter in yeast/glucose solution | Drop in pH as CO₂ forms carbonic acid |
| Calorimeter | Heat produced by respiring organisms |
📐 Calculating the Rate (Application of Skills)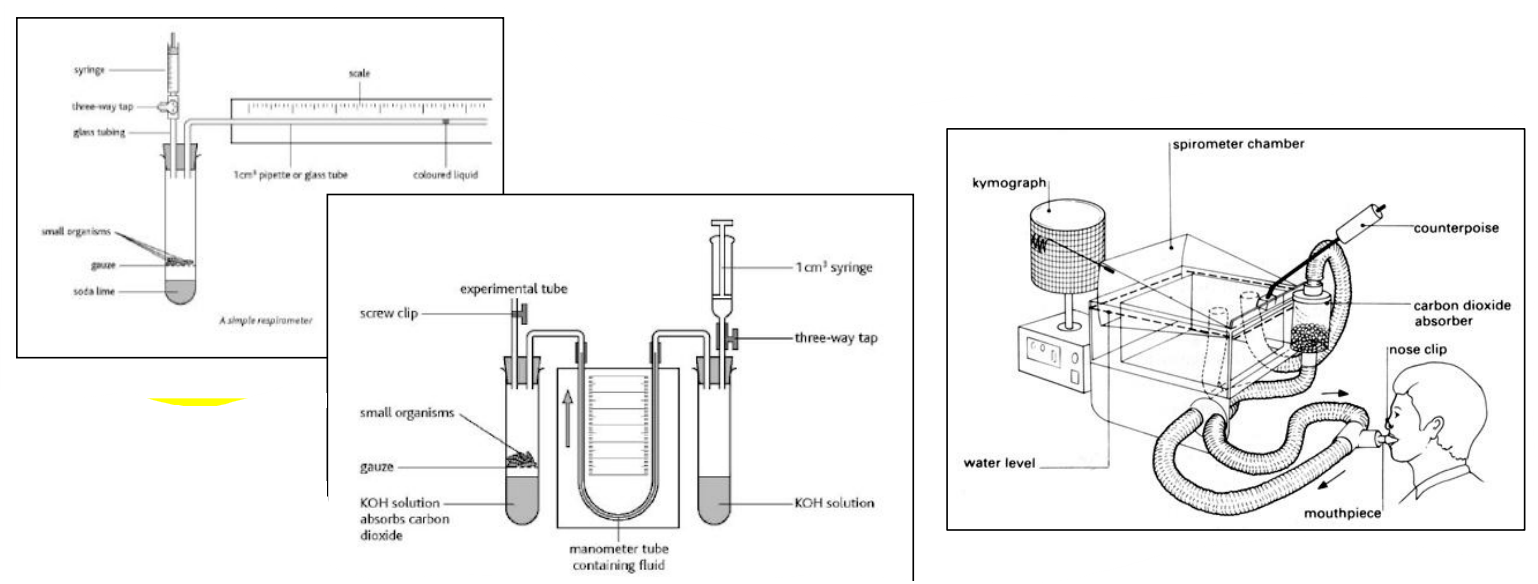
To calculate rate from data:
Rate = Change in amount (O₂ or CO₂) ÷ Time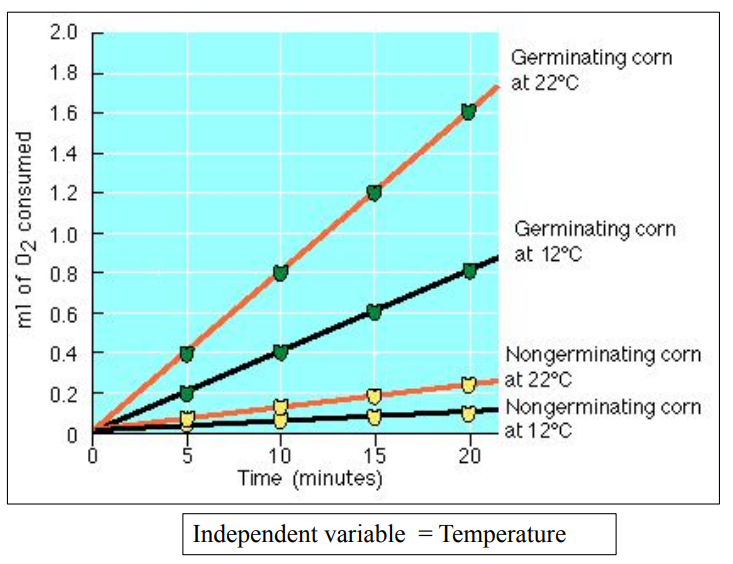
Example:
Oxygen used = 10 mL
Time = 5 minutes
Rate = 10 mL / 5 min = 2 mL/min
- Always include: Units
- Controlled variables (e.g., temperature, volume of solution)
🔎 Sources of Error in Experiments
- Leaks in apparatus
- Temperature fluctuations
- Unequal size/age of organisms (in biological samples)
- Measurement inaccuracy (e.g., using a manual syringe vs. electronic sensors)
– Temperature, pH, and substrate concentration are major factors affecting respiration rate.
– Rates are often measured via oxygen use or carbon dioxide production.
– Students should be confident in calculating rates using simple formulas from experimental or secondary data.
– Always account for variables and potential sources of error when analyzing results.
Additional Higher Level
C1.2.7 – Role of NAD in Cell Respiration (Hydrogen Carrier & Redox Reactions)
🧬 What Is NAD?
NAD stands for Nicotinamide Adenine Dinucleotide.
It’s a coenzyme used in cell respiration to carry hydrogen atoms (and their electrons) from one reaction to another.
Exists in two forms:
- NAD⁺ → oxidized form (ready to accept hydrogen)
- NADH → reduced form (carrying hydrogen)
🔁 Redox Reactions in Respiration
| Term | Definition |
|---|---|
| Oxidation | Loss of electrons or hydrogen (often via dehydrogenation) |
| Reduction | Gain of electrons or hydrogen |
| Redox reaction | A reaction where one substance is oxidized and another is reduced |
- ➡ In cell respiration, oxidation = removal of H (with electrons)
- ➡ This process is usually carried out by enzymes called dehydrogenases
⚙️ NAD’s Role in Respiration
- Picks up hydrogen atoms that are removed from substrates (e.g., glucose intermediates)
- Accepts 1 H⁺ ion and 2 electrons → becomes NADH
- NADH carries these electrons to the electron transport chain in mitochondria
- In the final step, NADH is oxidized back to NAD⁺, releasing energy to make ATP
Example Reaction:
NAD⁺ + 2H → NADH + H⁺
🔋 Why Is NAD Important?
| Function | Why It Matters |
|---|---|
| Transfers electrons & hydrogen | Essential for making ATP in respiration |
| Couples oxidation and reduction | Connects energy release from fuel to ATP production |
| Regenerates constantly | Allows continuous metabolism—if NAD⁺ isn’t regenerated, respiration stops |
Quick Visual: Oxidation by Dehydrogenation
| Substrate | Enzyme | Hydrogen Removed? | NAD Involved? | Result |
|---|---|---|---|---|
| Glucose | Dehydrogenase | Yes | Yes | NADH + oxidized intermediate |
– Oxidation in respiration = removal of hydrogen (and electrons) = dehydrogenation.
– NAD⁺ accepts hydrogen → becomes NADH, which carries electrons to the electron transport chain.
– NAD’s role in redox reactions is essential for ATP production and keeping respiration going.
C1.2.8 – Glycolysis: Conversion of Glucose to Pyruvate
🔍 What Is Glycolysis?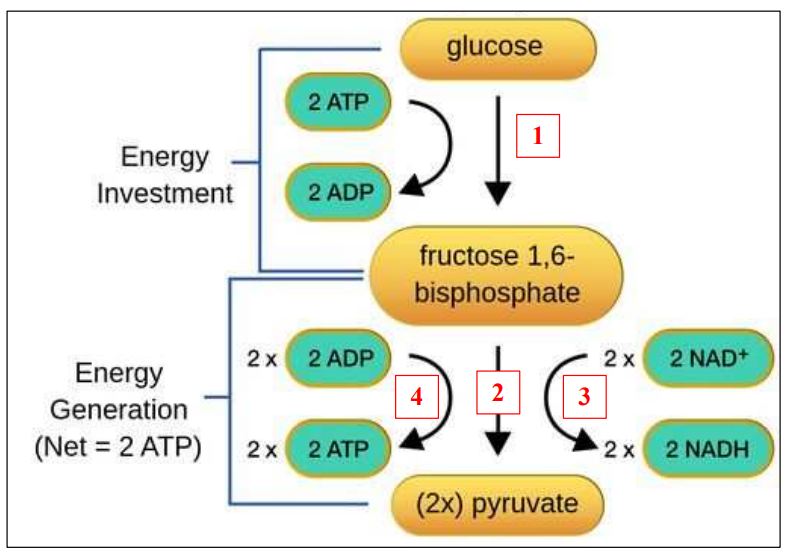
Glycolysis is the first stage of cellular respiration, happening in the cytoplasm of cells.
It breaks down glucose (6-carbon) into two pyruvate molecules (3-carbon).
It’s a stepwise, enzyme-controlled pathway with a net gain of:
- 2 ATP molecules
- 2 NADH molecules (reduced NAD)
🔬 Main Stages of Glycolysis
| Stage | What Happens | Purpose |
|---|---|---|
| 1. Phosphorylation | Glucose is activated by the addition of 2 phosphate groups from ATP | Makes glucose more reactive |
| 2. Lysis | The phosphorylated glucose splits into two 3-carbon sugars | Prepares for energy extraction |
| 3. Oxidation | Each 3-carbon molecule is oxidized — H atoms are removed and transferred to NAD⁺ → NADH | Captures energy-rich electrons |
| 4. ATP Formation | 4 ATP molecules are produced (2 per 3-carbon sugar) | 2 are used earlier → net gain = 2 ATP |
Note: Each of these steps is catalysed by a specific enzyme.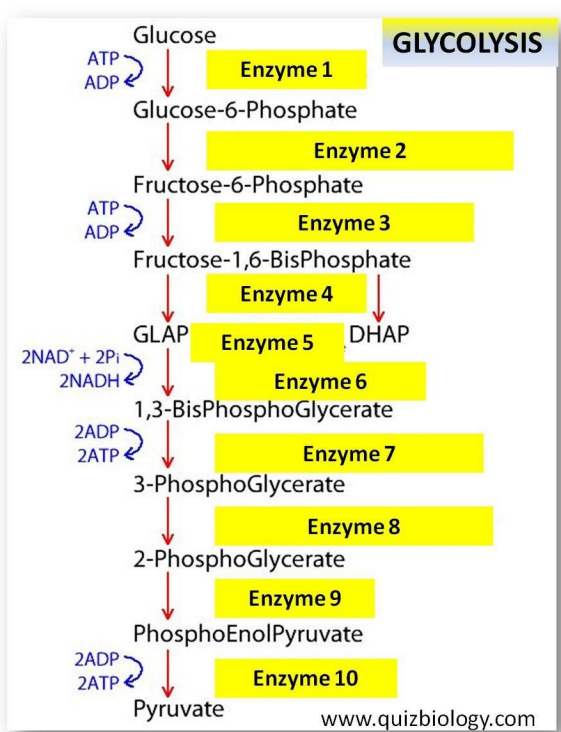
⚙️ Overview of Energy and Products
| Molecule | Produced in Glycolysis |
|---|---|
| ATP | 4 made – 2 used = 2 net |
| NADH | 2 reduced NAD (NADH) |
| Pyruvate | 2 molecules |
🧪 Enzymes in Glycolysis
- Glycolysis involves a chain of enzyme-catalysed reactions.
- Each step is controlled by a different enzyme, ensuring regulation and accuracy.
- This makes glycolysis efficient and tightly regulated.
– Glycolysis occurs in the cytoplasm without oxygen.
– Steps include: phosphorylation, lysis, oxidation, and ATP formation.
– Net yield: 2 ATP, 2 NADH, 2 pyruvate.
– Each reaction is catalysed by a different enzyme, making the process highly regulated.
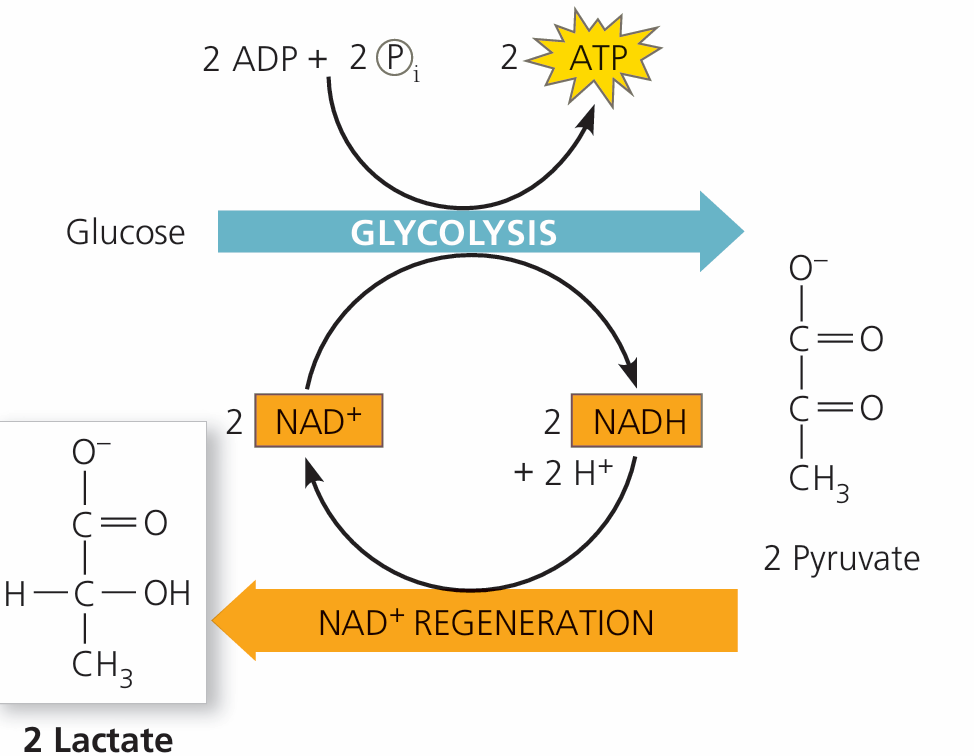
C1.2.9 – Conversion of Pyruvate to Lactate in Anaerobic Respiration
🧪 What Happens During Anaerobic Respiration in Humans?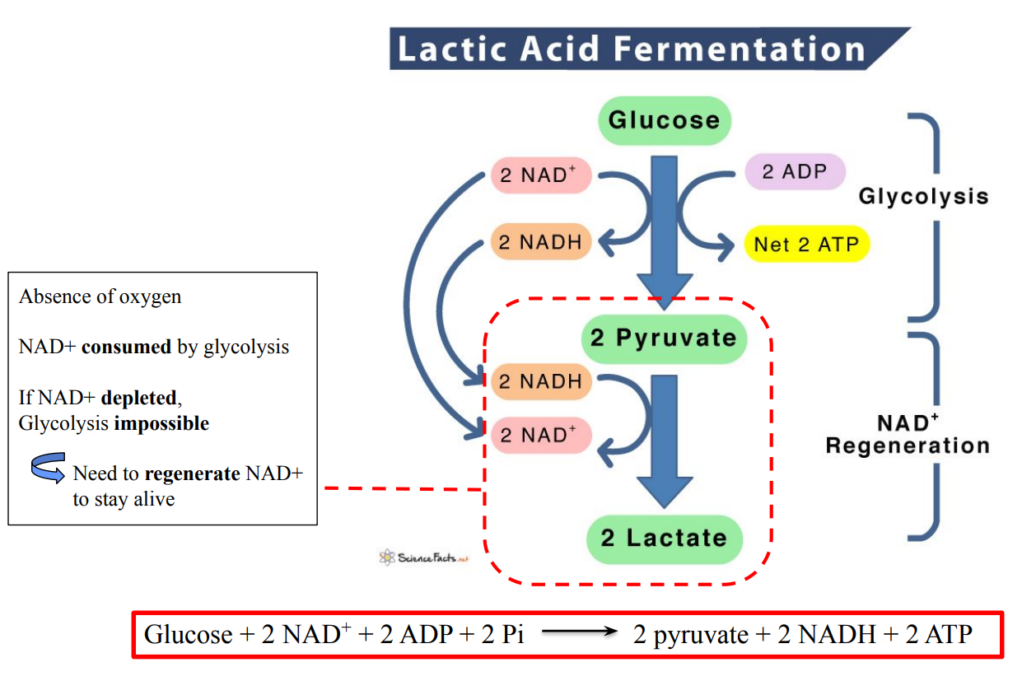
In low oxygen conditions (e.g. during intense exercise), cells switch to anaerobic respiration.
Glycolysis still occurs, converting glucose to pyruvate and producing:
- 2 ATP (net)
- 2 NADH
BUT… without oxygen, NADH can’t offload its hydrogen via the electron transport chain.
🔁 Solution: Convert Pyruvate → Lactate
| Step | Purpose |
|---|---|
| Pyruvate is reduced to lactate | Accepts hydrogen from NADH (via lactate dehydrogenase) |
| NADH is oxidized to NAD⁺ | Regenerates NAD⁺ so glycolysis can continue |
This process is called lactic acid fermentation.
It occurs in the cytoplasm of cells.
⚡ Why Is NAD⁺ Regeneration Important?
- Glycolysis depends on available NAD⁺ to accept hydrogen during oxidation steps.
- Without regenerating NAD⁺, glycolysis would stop, and ATP production halts.
- Regeneration ensures a continuous (although limited) supply of ATP under anaerobic conditions.
⚠️ Limitations
- Lactate buildup can lead to muscle fatigue and soreness.
- Lactate must be broken down later in the liver (Cori cycle), requiring oxygen.
– In anaerobic respiration, pyruvate is converted to lactate.
– This regenerates NAD⁺, allowing glycolysis to continue.
– Net gain: 2 ATP per glucose molecule – enough for short bursts of energy.
– Occurs in the cytoplasm, without the use of oxygen.
C1.2.10 – Anaerobic Cell Respiration in Yeast (Fermentation)
🧫 What Is Anaerobic Respiration in Yeast?
Like human cells, yeast cells can respire anaerobically when oxygen is absent.
Glycolysis occurs first:
Glucose → 2 pyruvate
Produces 2 ATP (net) and 2 NADH
BUT… the key difference is how NAD⁺ is regenerated.
🔄 How Yeast Regenerates NAD⁺
| Organism | Pyruvate is converted to… | Final products |
|---|---|---|
| Humans | Lactate | Lactate (lactic acid) |
| Yeast | Ethanol + CO₂ | Ethanol (alcohol) + carbon dioxide |
In yeast: 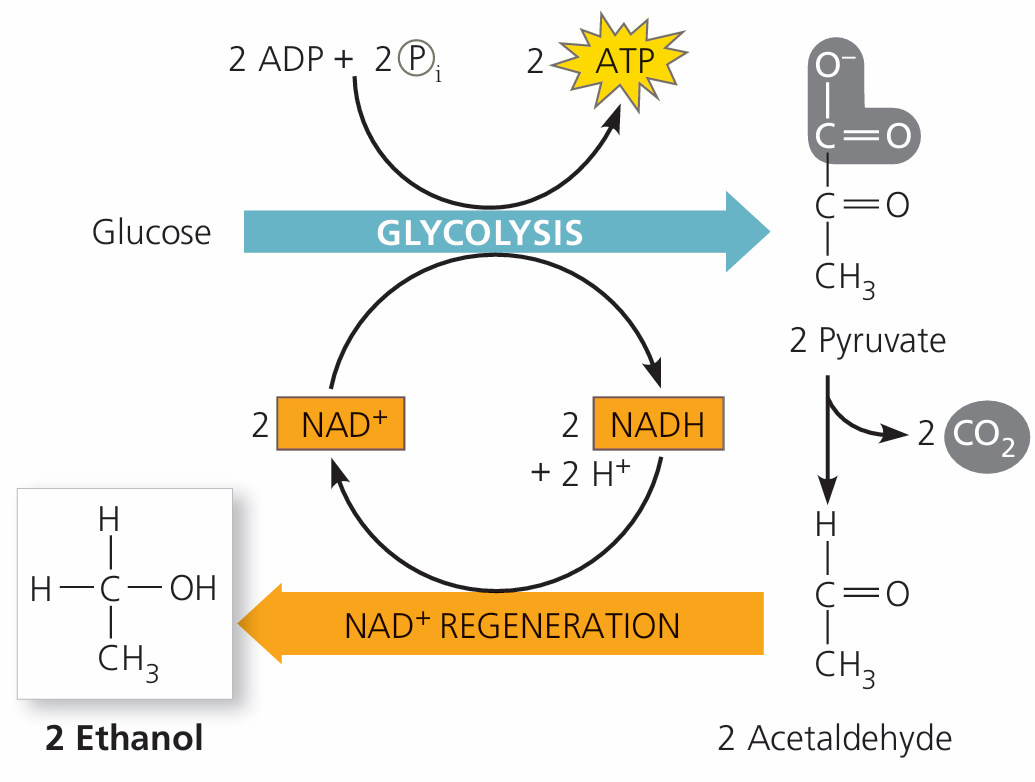
- Pyruvate → CO₂ + Acetaldehyde
- Acetaldehyde + NADH → Ethanol + NAD⁺
This regenerates NAD⁺ so glycolysis can continue.
Uses of Anaerobic Respiration in Industry
1. Brewing (Alcohol Production)
- Yeast is added to sugar solutions (like fruit juice or grain mash).
- In the absence of oxygen, yeast:
- Produces ethanol (alcohol)

- Releases carbon dioxide
- Produces ethanol (alcohol)
- Used in beer, wine, and spirits production.
2. Baking
- Yeast is mixed with dough.
- During fermentation:
- CO₂ forms bubbles → dough rises
- Ethanol evaporates during baking
- Gives bread its light texture.
🔬 Fermentation Summary
| Step | Molecules Involved |
|---|---|
| Glucose → Pyruvate | Glycolysis (produces 2 ATP, 2 NADH) |
| Pyruvate → CO₂ + Ethanol | Regenerates NAD⁺ from NADH |
| NAD⁺ → Back to glycolysis | Glycolysis continues to make more ATP |
– Yeast performs anaerobic respiration when oxygen is lacking.
– It produces ethanol and carbon dioxide, unlike humans who produce lactate.
– This process is used in brewing (alcohol) and baking (bread rise).
– The main goal is to regenerate NAD⁺ so glycolysis (and ATP production) can continue.
C1.2.11 – Link Reaction: Oxidation & Decarboxylation of Pyruvate
🧬 What Is the Link Reaction?
The link reaction connects glycolysis to the Krebs cycle.
Occurs in the mitochondrial matrix, but only in the presence of oxygen (aerobic conditions).
It’s called a “link” because it links the cytoplasmic process (glycolysis) with mitochondrial respiration.
🔄 Step-by-Step Breakdown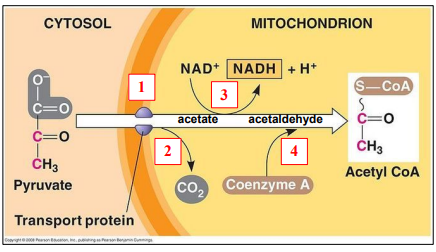
🧪 What happens to pyruvate (3C)?
- Decarboxylation: One carbon is removed → CO₂ released
- Pyruvate (3C) → Acetyl group (2C)
- Oxidation: Pyruvate loses hydrogen atoms
- Hydrogen is accepted by NAD⁺ → NADH
- Coenzyme A (CoA): Acetyl group (2C) is attached to CoA → forms Acetyl-CoA
Acetyl-CoA then enters the Krebs cycle.
🧬 What about lipids and other molecules?
- Carbohydrates like glucose are broken into pyruvate, then into acetyl-CoA.
- Lipids (fats) are also broken into acetyl groups.
- These acetyl groups bind to Coenzyme A.
- So, fats can also “feed into” the Krebs cycle!
🔁 Link Reaction Summary
| Reactant | Process | Products |
|---|---|---|
| Pyruvate (3C) | Decarboxylation | CO₂ (1C lost) |
| Pyruvate | Oxidation | NAD⁺ → NADH |
| Acetyl group | Addition of CoA | Acetyl-CoA (2C) |
Occurs once per pyruvate, so twice per glucose molecule.
💡 Why It Matters
- Acetyl-CoA is the fuel for the Krebs cycle.
- NADH carries high-energy electrons to the electron transport chain.
- This step is essential for extracting maximum ATP from glucose or fat.
– The link reaction converts pyruvate (3C) to acetyl-CoA (2C) via decarboxylation and oxidation.
– CO₂ is released and NADH is produced.
– Acetyl groups from both carbohydrates and lipids enter the Krebs cycle by binding to Coenzyme A.
– This step is aerobic and occurs in the mitochondrial matrix.
C1.2.12 – The Krebs Cycle (Citric Acid Cycle)
🧬 What Is the Krebs Cycle?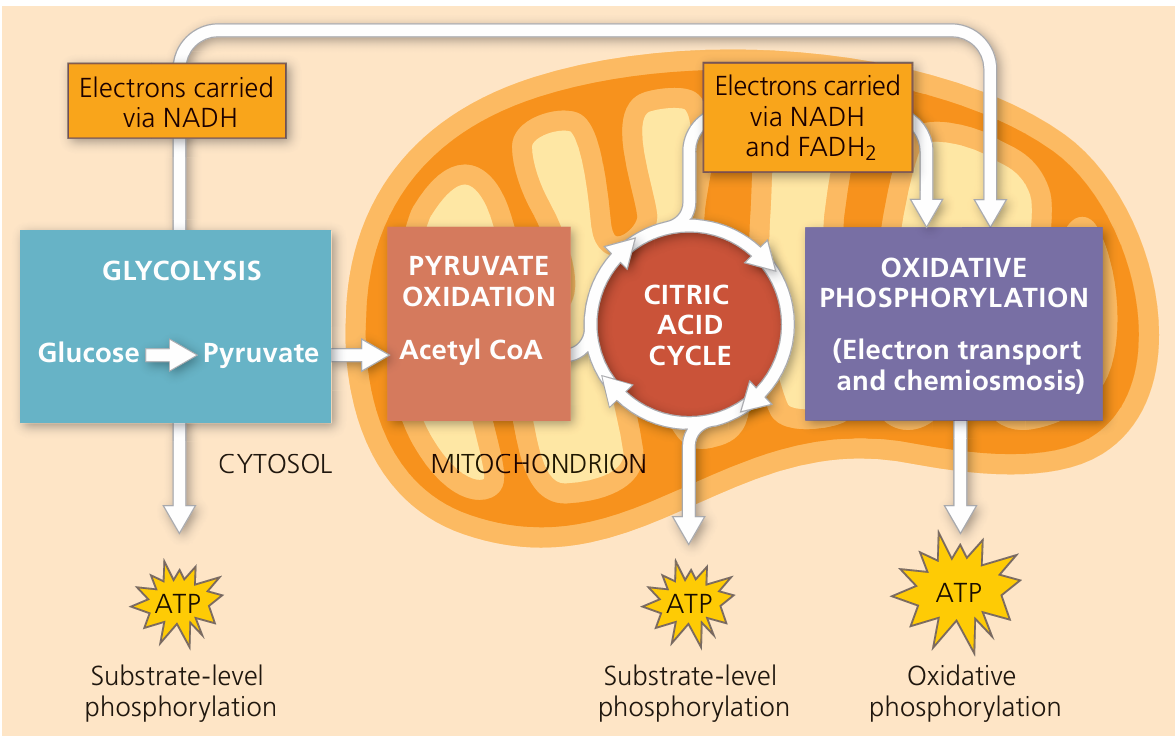
A cyclical pathway of reactions inside the mitochondrial matrix.
It oxidizes acetyl-CoA to release energy.
Named after Hans Krebs, who discovered it.
Happens only in aerobic respiration.
🔄 Main Players You Need to Know
| Molecule | Carbon Count | Role |
|---|---|---|
| Acetyl-CoA | 2C | Enters the cycle |
| Citrate | 6C | First intermediate formed |
| Oxaloacetate | 4C | Combines with acetyl group, regenerated |
⚙️ Step-by-Step Overview
- Acetyl-CoA (2C) combines with oxaloacetate (4C) → forms citrate (6C)
- Citrate undergoes a series of reactions that:
- Release 2 CO₂ molecules (decarboxylations)
- Perform 4 oxidations (dehydrogenation reactions)
- Regenerate oxaloacetate (4C) to restart the cycle
⚡ What Is Produced Per Acetyl-CoA?
| Product | Function |
|---|---|
| 2 CO₂ | Waste product (from decarboxylation) |
| 3 NADH | Carries electrons to the electron transport chain |
| 1 FADH₂ | Also carries electrons |
| 1 ATP (or GTP) | Usable energy |
| Oxaloacetate | Recycled for the next cycle |
Since 1 glucose = 2 acetyl-CoA → everything above happens twice per glucose!
🔥 Oxidation = Dehydrogenation
- Oxidation means removing hydrogen atoms from intermediates.
- These hydrogens are accepted by:
- NAD⁺ → NADH
- FAD → FADH₂
– The Krebs cycle starts when acetyl-CoA (2C) joins oxaloacetate (4C) → forms citrate (6C).
– Oxaloacetate is regenerated, making it a cyclical process.
– Includes 4 oxidations (dehydrogenation) and 2 decarboxylations.
– Produces NADH, FADH₂, CO₂, and 1 ATP per acetyl-CoA.
– Occurs in the mitochondrial matrix and only during aerobic respiration.
C1.2.13 – Transfer of Energy by Reduced NAD to the Electron Transport Chain
🧬 What Is Reduced NAD?
NAD = Nicotinamide Adenine Dinucleotide
It’s a coenzyme that carries electrons and hydrogen.
When it gains hydrogen (H), it becomes reduced NAD (NADH).
This includes a pair of high-energy electrons.
🏭 Where Does NADH Come From?
| Process | Location | What It Produces |
|---|---|---|
| Glycolysis | Cytoplasm | 2 NADH per glucose |
| Link Reaction | Mitochondrial matrix | 2 NADH per glucose |
| Krebs Cycle | Mitochondrial matrix | 6 NADH per glucose |
Total from one glucose = 10 NADH
🧱 The Electron Transport Chain (ETC)
Located in the inner mitochondrial membrane
A series of protein carriers (enzymes) that pass electrons down a chain.
Electrons move from high → low energy, releasing energy step-by-step.
⚙️ How NADH Transfers Energy
- NADH donates a pair of electrons to the first carrier in the ETC.
- This oxidizes NADH → NAD⁺, which can be reused.
- Electrons move along the chain, releasing energy at each step.
- This energy is used to pump H⁺ ions (protons) into the intermembrane space.
- This sets up a proton gradient needed for ATP synthesis via chemiosmosis.
🔁 Why Is This Important?
- The ETC is the main source of ATP in aerobic respiration.
- Reduced NAD = essential energy carrier connecting earlier stages (glycolysis, link, Krebs) to the final ATP production.
– Reduced NAD (NADH) carries energy-rich electrons and hydrogen.
– It’s formed during glycolysis, link reaction, and the Krebs cycle.
– In the mitochondria, NADH donates electrons to the electron transport chain.
– This transfers energy helps pump protons, and powers ATP production.
– NAD⁺ is recycled for more reactions.
C1.2.14 – Generation of a Proton Gradient by Flow of Electrons Along the Electron Transport Chain
🧬 What Is a Proton Gradient?
- A proton gradient means there’s a difference in H⁺ (proton) concentration across a membrane.
- In mitochondria, it forms across the inner mitochondrial membrane.
- It’s essential for ATP production during aerobic respiration.
⚙️ How the Proton Gradient Is Made
- Reduced NAD (NADH) and reduced FAD (FADH₂) donate electrons to the electron transport chain (ETC).
- As electrons flow along the ETC:
- They release energy.
- This energy is used to pump protons (H⁺) from the matrix → intermembrane space.
- The result is a high concentration of protons in the intermembrane space and a low concentration in the matrix.
This is the proton gradient (also called an electrochemical gradient).
📉 Why It Matters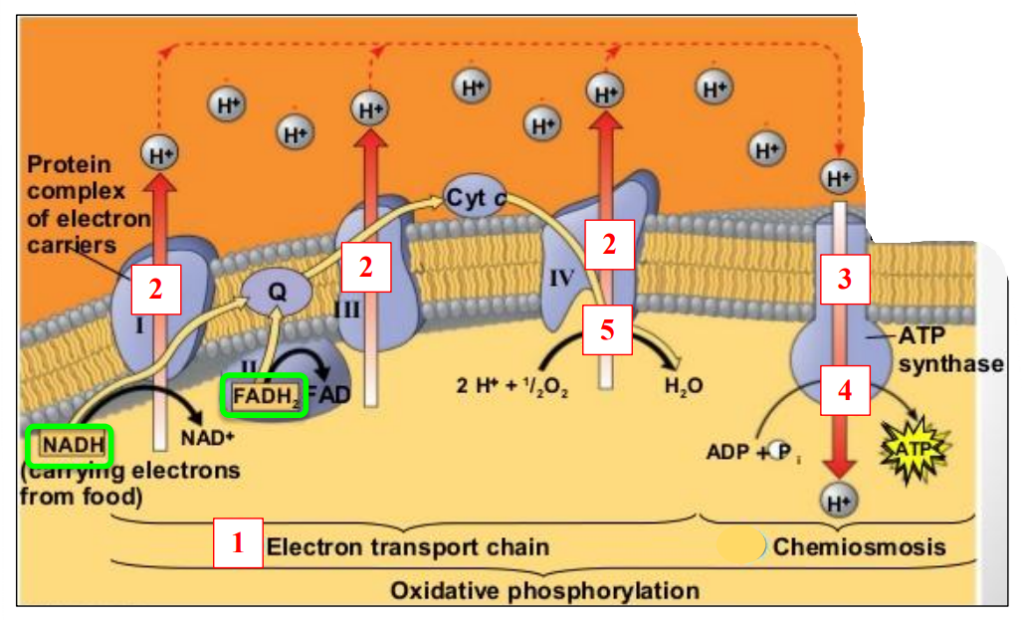
- The proton gradient stores potential energy.
- Protons naturally want to flow back into the matrix, but they must go through ATP synthase.
- When they do, ATP is produced from ADP + phosphate.
This process is called chemiosmosis.
🧠 Remember
- Electrons provide the energy to pump protons.
- The ETC doesn’t make ATP directly – it just builds the gradient that allows ATP synthase to do it.
– Electrons from NADH and FADH₂ flow through the electron transport chain (ETC).
– Their energy is used to pump protons (H⁺) across the inner mitochondrial membrane.
– This creates a proton gradient, which stores energy.
– The gradient drives ATP synthesis through chemiosmosis.
– This is a key step in aerobic respiration.
C1.2.15 – Chemiosmosis and the Synthesis of ATP in the Mitochondrion
🌀 What Is Chemiosmosis?
Chemiosmosis is the movement of protons (H⁺) down their concentration gradient across a membrane through a special enzyme – ATP synthase.
It links the energy released by the electron transport chain (ETC) to the production of ATP.
🧪 Where It Happens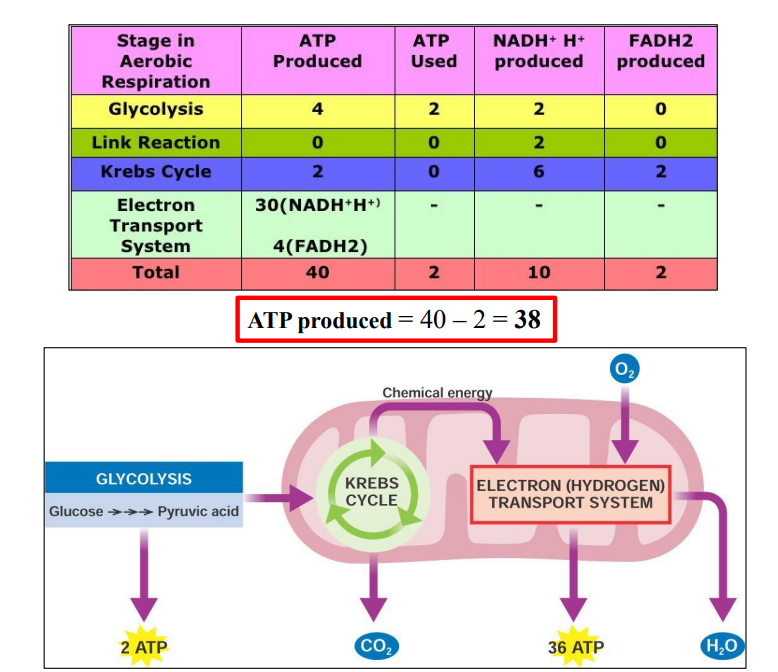
- Inside the mitochondrion, specifically:
- Across the inner mitochondrial membrane
- Between the intermembrane space and matrix
⚙️ How ATP Is Made from the Proton Gradient
- The ETC pumps H⁺ ions into the intermembrane space, creating a proton gradient.
- Protons flow back into the matrix through ATP synthase (a membrane protein).
- The energy from this proton movement is used by ATP synthase to:
- Add a phosphate group to ADP
- Form ATP (adenosine triphosphate)
This process is called oxidative phosphorylation.
🔄 Coupling Explained
ATP synthase acts like a molecular motor powered by proton flow.
It couples the energy from H⁺ movement to the phosphorylation of ADP, forming ATP.
🧠 Remember
- Chemiosmosis = H⁺ flow + ATP production
- It’s the final stage of aerobic respiration
- Most of the cell’s ATP is made this way
🧩 Simple Analogy
Think of the proton gradient as water behind a dam and ATP synthase as a turbine.
As the water (H⁺) flows through, it spins the turbine (ATP synthase), generating electricity (ATP) ⚡
– Chemiosmosis is the movement of protons (H⁺) down a gradient via ATP synthase.
– This movement releases energy, used to convert ADP + phosphate → ATP.
– It happens in the inner mitochondrial membrane.
– This is the main way ATP is made in aerobic respiration.
– The process is called oxidative phosphorylation.
C1.2.16 – Role of Oxygen as Terminal Electron Acceptor in Aerobic Cell Respiration
🧪 What Happens in the Electron Transport Chain (ETC)?
As electrons move through the ETC in the inner mitochondrial membrane, they lose energy.
This energy is used to pump protons (H⁺) into the intermembrane space, building a proton gradient (→ chemiosmosis).
🌬️ The Vital Role of Oxygen
Oxygen (O₂) is the terminal electron acceptor – the final destination for the electrons.
At the end of the ETC:
- O₂ combines with electrons (e⁻) coming from the ETC
- And with protons (H⁺) from the matrix
- Forming metabolic water (H₂O)
⚡ Why Oxygen Matters:
- If O₂ isn’t present:
- Electrons have nowhere to go – they back up the ETC
- The ETC stops, so no more proton pumping
- ATP production halts
- Oxygen keeps the flow of electrons continuous, maintaining:
- The proton gradient
- Chemiosmosis
- ATP synthesis
🧬 Simple Word Equation:
O₂ + 4e⁻ + 4H⁺ → 2H₂O
– Oxygen is the final electron acceptor in the ETC.
– It combines with electrons and protons to form metabolic water.
– This step keeps the ETC functioning and allows continued ATP production.
– Without oxygen, aerobic respiration halts, and the cell may switch to anaerobic pathways.
C1.2.17 – Differences Between Lipids and Carbohydrates as Respiratory Substrates
🧬 What Are Respiratory Substrates?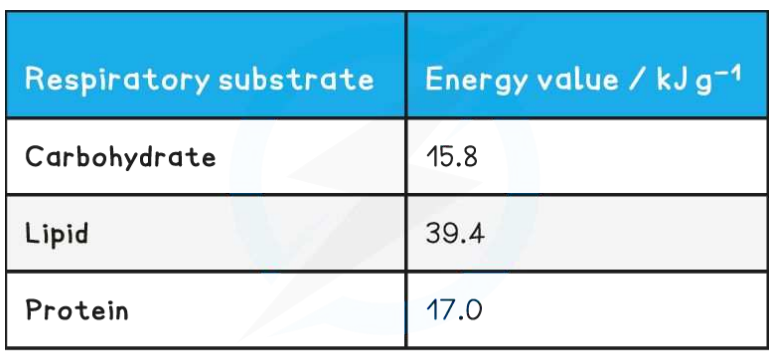
These are molecules used to release energy in cell respiration.
The two main types:
- Carbohydrates (like glucose)
- Lipids (like triglycerides → fatty acids)
🔍 Comparison: Lipids vs. Carbohydrates
| Feature | Carbohydrates | Lipids |
|---|---|---|
| Energy yield per gram | Lower | Higher |
| Oxygen content | Higher | Lower |
| Hydrogen and carbon | Less oxidizable | More oxidizable |
| Pathway entry | Glycolysis → Pyruvate → Acetyl-CoA | Fatty acids → 2C acetyl groups → Acetyl-CoA |
| Used in anaerobic respiration? | Yes | No |
| Used in glycolysis? | Yes | No |
🔥 Why Lipids Give More Energy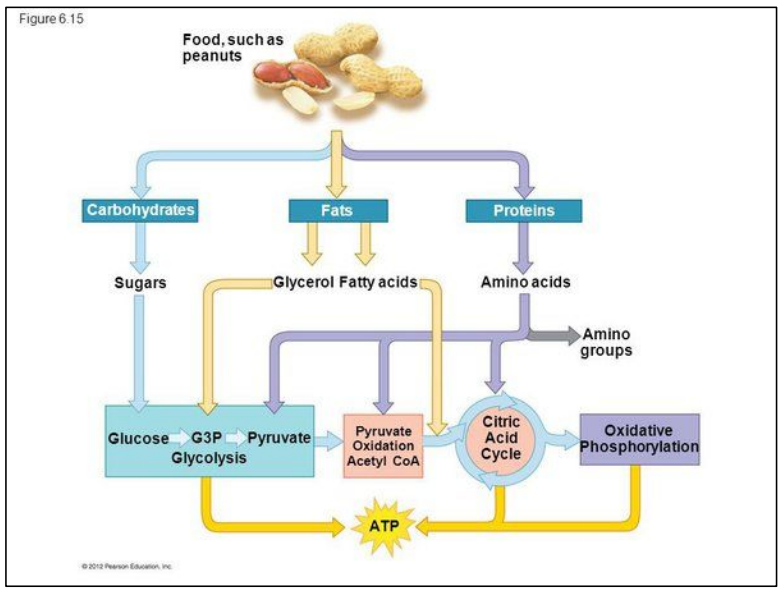
- Lipids have more C-H bonds, which are oxidized during respiration.
- They are less oxygenated → more energy can be released when oxygen is added.
- Therefore, lipids yield more ATP per gram than carbohydrates.
🧪 What Happens During Lipid Respiration?
- Lipids are broken down into fatty acids.
- Fatty acids are converted into 2-carbon (2C) acetyl groups.
- These enter the Krebs cycle via acetyl-CoA (acetyl coenzyme A).
Important Note on Anaerobic Respiration
- Only carbohydrates can go through glycolysis and anaerobic respiration.
- Lipids require oxygen – they cannot be metabolized anaerobically.
– Lipids provide more energy per gram due to more oxidizable C and H.
– Only carbohydrates can be used in glycolysis and anaerobic respiration.
– Fatty acids enter via acetyl-CoA, bypassing glycolysis.
– Lipid respiration is strictly aerobic.