IB DP Biology D1.2 Protein synthesis Study Notes - New Syllabus -2025
IB DP Biology D1.2 Protein synthesis Study Notes – New syllabus 2025
IB DP Biology D1.2 Protein synthesis Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on IB Biology syllabus with guiding questions of
- How does a cell produce a sequence of amino acids from a sequence of DNA bases?
- How is the reliability of protein synthesis ensured?
Standard level and higher level: 3 hours
Additional higher level: 3 hours
D1.2.1 – Transcription: Synthesis of RNA Using a DNA Template
🔍 What is Transcription?
Transcription is the process of making RNA from a DNA template.
It is the first step in gene expression, leading to protein synthesis.
🛠️ Role of RNA Polymerase
- RNA polymerase binds to the promoter region on DNA.
- Unwinds the DNA strands.
- Adds RNA nucleotides complementary to the DNA template strand.
- Synthesizes RNA in the 5’ to 3’ direction.
- Only the template DNA strand is copied.
🧩 Result of Transcription
Produces a single-stranded messenger RNA (mRNA) molecule.
mRNA carries the genetic code from DNA to the ribosome for protein synthesis.
🔑 Key Points
- Transcription copies DNA into RNA.
- RNA polymerase controls the process.
- Only a gene (section of DNA) is transcribed.
Transcription is the process where RNA polymerase synthesizes RNA by copying one DNA strand, producing mRNA that carries genetic information for protein synthesis.
D1.2.2 – Role of Hydrogen Bonding & Complementary Base Pairing in Transcription
🔍 Complementary Base Pairing in Transcription
RNA polymerase builds the RNA strand using the DNA template strand.
Each RNA nucleotide added is complementary to the DNA base it pairs with.
Base pairing rules during transcription:
- Adenine (A) on DNA pairs with Uracil (U) on RNA
- Thymine (T) on DNA pairs with Adenine (A) on RNA
- Guanine (G) on DNA pairs with Cytosine (C) on RNA
- Cytosine (C) on DNA pairs with Guanine (G) on RNA
🔗 Hydrogen Bonding
Complementary bases pair via hydrogen bonds, stabilizing the RNA-DNA hybrid during transcription.
Only specific base pairs form hydrogen bonds, ensuring accuracy.
🧬 Sense and Template Strands
- The template strand (antisense strand) is the DNA strand copied to make RNA.
- The sense strand (coding strand) has the same base sequence as the RNA produced, except uracil replaces thymine.
- Transcription copies the template strand’s sequence into RNA, producing an RNA strand complementary to the template and matching the sense strand sequence.
🔑 Why It Matters
This precise base pairing mechanism ensures the correct genetic code is transcribed.
Hydrogen bonds allow temporary attachment without permanent change to DNA.
During transcription, complementary base pairing (A with U, T with A, G with C, C with G) and hydrogen bonding enable accurate RNA synthesis using the DNA template strand.
D1.2.3 – Stability of DNA Templates
🔍 Why DNA Template Stability Matters
- During transcription, single DNA strands are temporarily separated to serve as templates.
- The original DNA base sequence must remain unchanged to ensure accurate genetic information.
- After RNA polymerase passes, the DNA strands rejoin via hydrogen bonding.
⏳ Minimizing Mutation Risks
- DNA strands are separated only briefly during transcription.
- This short exposure reduces the chance of chemical damage or mutations.
- Maintaining DNA integrity is critical, especially in non-dividing somatic cells that use the same DNA throughout their life.
⚠️ Consequences of Template Instability
Frequent mutations in the DNA template would cause errors in:
- RNA transcripts (wrong base sequences)
- Protein synthesis (wrong amino acids)
- Such mistakes can impair protein function, affecting cell health.
🔑 Key Takeaway
DNA template stability is essential for accurate transcription and proper cell function.
The cell’s mechanisms ensure minimal change to the DNA sequence despite repeated use.
DNA templates remain stable during transcription by briefly separating strands and quickly reforming hydrogen bonds, preventing mutations and ensuring accurate RNA and protein synthesis.
D1.2.4 – Transcription as a Process Required for Gene Expression
🔍 What is Gene Expression?
Gene expression is the process by which information from a gene is used to make a functional product, usually a protein.
Transcription is the first and crucial step in gene expression.
🔑 Control of Gene Expression
- Not all genes in a cell are active (expressed) at the same time.
- Cells regulate gene expression mainly by switching transcription on or off.
- When a gene is switched on, its DNA is transcribed into RNA.
- When a gene is switched off, transcription does not occur, so no RNA or protein is made.
⚙️ Why Control Transcription?
- Controls which proteins are produced according to the cell’s needs.
- Allows cells to respond to changes in the environment or developmental signals.
- Saves energy by only producing necessary proteins.
Transcription is the key first step in gene expression and is tightly regulated so that only certain genes are expressed at any time, allowing cells to control protein production.
D1.2.5 – Translation: Synthesis of Polypeptides from mRNA
🔍 What is Translation?
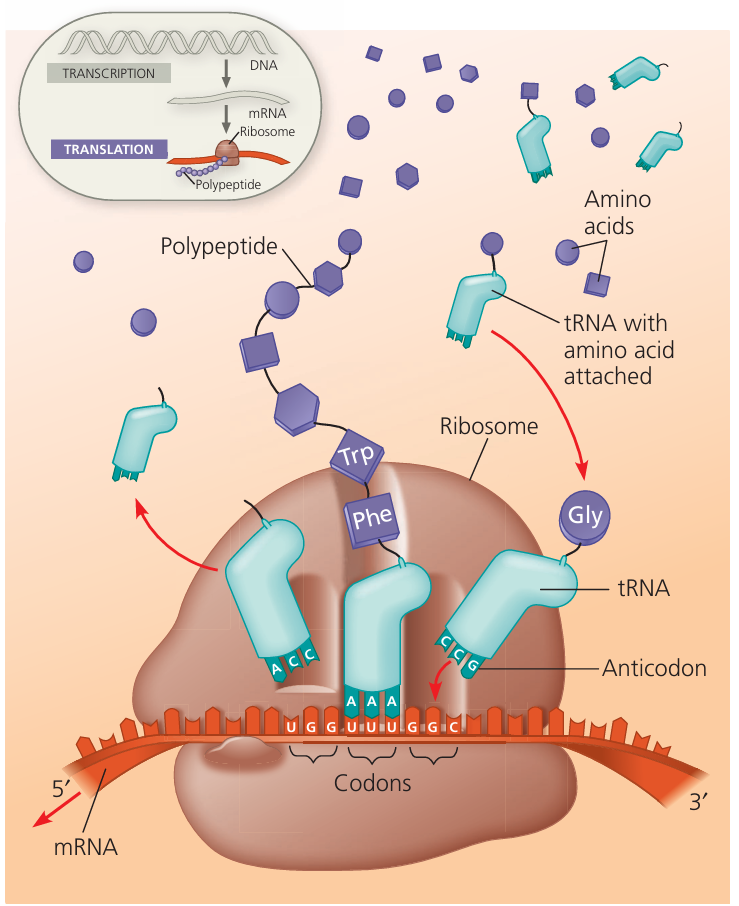
Translation is the process of building a polypeptide (protein) by reading the base sequence of mRNA.
The sequence of bases in mRNA determines the order of amino acids in the polypeptide.
🧩 How Does Translation Work?
- The genetic code on mRNA is read in groups of three bases called codons.
- Each codon corresponds to a specific amino acid.
- Amino acids are linked together in the exact order specified by the mRNA sequence to form a polypeptide chain.
🌍 Where Does Translation Occur?
Takes place in the cytoplasm.
- In eukaryotes, mRNA is produced in the nucleus and moves through nuclear pores into the cytoplasm.
- In prokaryotes, transcription and translation happen in the same area because there is no nucleus.
🔑 Role of mRNA
mRNA (messenger RNA) carries the genetic information copied from DNA.
It acts as a template for assembling amino acids into a polypeptide.
Translation converts the genetic code in mRNA into a specific sequence of amino acids, synthesizing polypeptides that form proteins, a key step in gene expression.
D1.2.6 – Roles of mRNA, Ribosomes, and tRNA in Translation
🔍 Key Players in Translation
| Component | Role in Translation |
|---|---|
| mRNA | Carries the genetic code (sequence of codons) from DNA to ribosome. Binds to the small subunit of the ribosome. |
| Ribosome | Site where translation happens. Has two subunits: small and large. The small subunit binds mRNA, and the large subunit has sites for tRNA binding and peptide bond formation. |
| tRNA | Transfers specific amino acids to the ribosome. Has an anticodon that pairs with the mRNA codon. Two tRNAs can bind simultaneously to the large subunit of the ribosome. |
⚙️ How They Work Together
- mRNA binds first to the small ribosomal subunit.
- The ribosome reads mRNA codons one at a time.
Two tRNA molecules attach to the large subunit at once:
- One holds the growing polypeptide chain.
- The other brings the next amino acid.
Peptide bonds form between amino acids, building the polypeptide.
Translation involves mRNA carrying the code to ribosomes where tRNA molecules bring amino acids to the large subunit, enabling the polypeptide chain to form.
D1.2.7 – Complementary Base Pairing Between tRNA and mRNA
🔍 Key Terms
- Codon: A sequence of three bases on the mRNA that codes for a specific amino acid.
- Anticodon: A complementary three-base sequence on the tRNA that pairs with the mRNA codon.
⚙️ How Base Pairing Works in Translation
- During translation, each tRNA molecule carries a specific amino acid.
- The anticodon of the tRNA binds to the complementary codon on the mRNA by base pairing.
- This pairing ensures that amino acids are added in the correct order dictated by the mRNA sequence.
🔄 Base Pairing Rules
| mRNA Codon Base | tRNA Anticodon Base |
|---|---|
| Adenine (A) | Uracil (U) |
| Uracil (U) | Adenine (A) |
| Cytosine (C) | Guanine (G) |
| Guanine (G) | Cytosine (C) |
Accurate matching between the mRNA codon and tRNA anticodon through complementary base pairing ensures the correct amino acid sequence during protein synthesis.
D1.2.8 – Features of the Genetic Code
🔍 Why a Triplet Code?
The genetic code uses 3 bases (a triplet) to code for one amino acid.
This is because:
– There are 4 different bases (A, T/U, C, G).
– If only 1 or 2 bases coded for amino acids, the number of possible amino acids would be too small.
– Triplets allow for 64 (4³) combinations, enough to code for all 20 amino acids plus stop signals.
📚 Important Terms
| Term | Meaning |
|---|---|
| Degeneracy | More than one codon can code for the same amino acid. For example, GGU, GGC, GGA, and GGG all code for glycine. This provides a safeguard against some mutations. |
| Universality | The genetic code is nearly the same in all living organisms, from bacteria to humans. This means that the same codon codes for the same amino acid across species, showing common ancestry. |
⚙️ Key Features of the Genetic Code
- Triplet: 3 bases per codon.
- Non-overlapping: Each base is part of only one codon.
- Degenerate: Multiple codons for many amino acids.
- Universal: The code is consistent across almost all organisms.
- Start and Stop signals: Specific codons signal the start (AUG) and stop of translation.
The triplet genetic code provides enough combinations to specify all amino acids. Its degeneracy offers protection from mutations, and its universality reflects the shared biology of life.
D1.2.9 – Using the Genetic Code Expressed as a Table of mRNA Codons
🔍 Reading the Genetic Code Table
- The genetic code is often shown as a codon table.
- Each codon is a sequence of three mRNA bases (A, U, C, G).
- By using this table, you can translate an mRNA sequence into an amino acid chain (polypeptide).
🧩 How to Deduce Amino Acid Sequence from mRNA
- Split the mRNA strand into groups of three bases (codons), starting from the start codon (AUG).
- Use the codon table to find the amino acid for each codon.
- Continue reading codons until a stop codon (UAA, UAG, or UGA) is reached.
- The sequence of amino acids you get is the primary structure of the protein.
🧬 Example
mRNA strand:
AUG – GGC – UUU – UAA
Using codon table:
AUG → Methionine (Start codon)
GGC → Glycine
UUU → Phenylalanine
UAA → Stop (translation ends here)
Polypeptide sequence: Methionine – Glycine – Phenylalanine
⚠️ Things to Remember
- Translation always starts at the start codon (AUG).
- Stop codons do not code for any amino acid; they signal translation to stop.
- Each codon corresponds to one amino acid only.
Use the mRNA codon table to convert codons into amino acids, reading from start to stop codons to deduce the amino acid sequence of the polypeptide.
D1.2.10 – Stepwise Movement of the Ribosome Along mRNA and Peptide Bond Formation
🔑 Elongation of the Polypeptide Chain
Translation proceeds through a repeating cycle that adds one amino acid at a time to the growing polypeptide.
🔄 Cycle Steps
- Amino acid attachment to tRNA: An activating enzyme attaches a specific amino acid to its matching tRNA, based on the anticodon.
- tRNA binds at the A site: The tRNA carrying the amino acid binds to the A (aminoacyl) site of the ribosome. The anticodon of the tRNA pairs with the complementary codon on the mRNA.
- Peptide bond formation: The amino acid on the tRNA in the A site is linked by a peptide bond to the growing polypeptide chain held by the tRNA at the P (peptidyl) site.
- Ribosome moves along mRNA: The ribosome shifts three bases (one codon) along the mRNA. The tRNA holding the growing polypeptide moves from the A site to the P site. The anticodon remains paired with the codon on the mRNA.
- Polypeptide transfer: The polypeptide is transferred to the newly arrived tRNA in the A site, which carries the next amino acid.
- tRNA exit: The now empty tRNA moves from the P site to the E (exit) site and leaves the ribosome, breaking its anticodon-codon pairing.
🔄 Cycle repeats: The process repeats with a new amino acid being attached to a tRNA and entering the A site, elongating the polypeptide step by step.
D1.2.11 – Mutations That Change Protein Structure
🔍 What is a Mutation?
A mutation is a change in the DNA base sequence.
This change can alter the sequence of amino acids in a protein, potentially changing its structure and function.
🧩 Types of Mutations Affecting Protein Structure
Point mutations: Changes to a single base in the DNA sequence.
These mutations can lead to:
- Missense mutation: One amino acid is swapped for another.
- Nonsense mutation: A stop codon is introduced prematurely, truncating the protein.
- Silent mutation: No change to the amino acid (due to degeneracy of the code).
📌 Example: Sickle Cell Anemia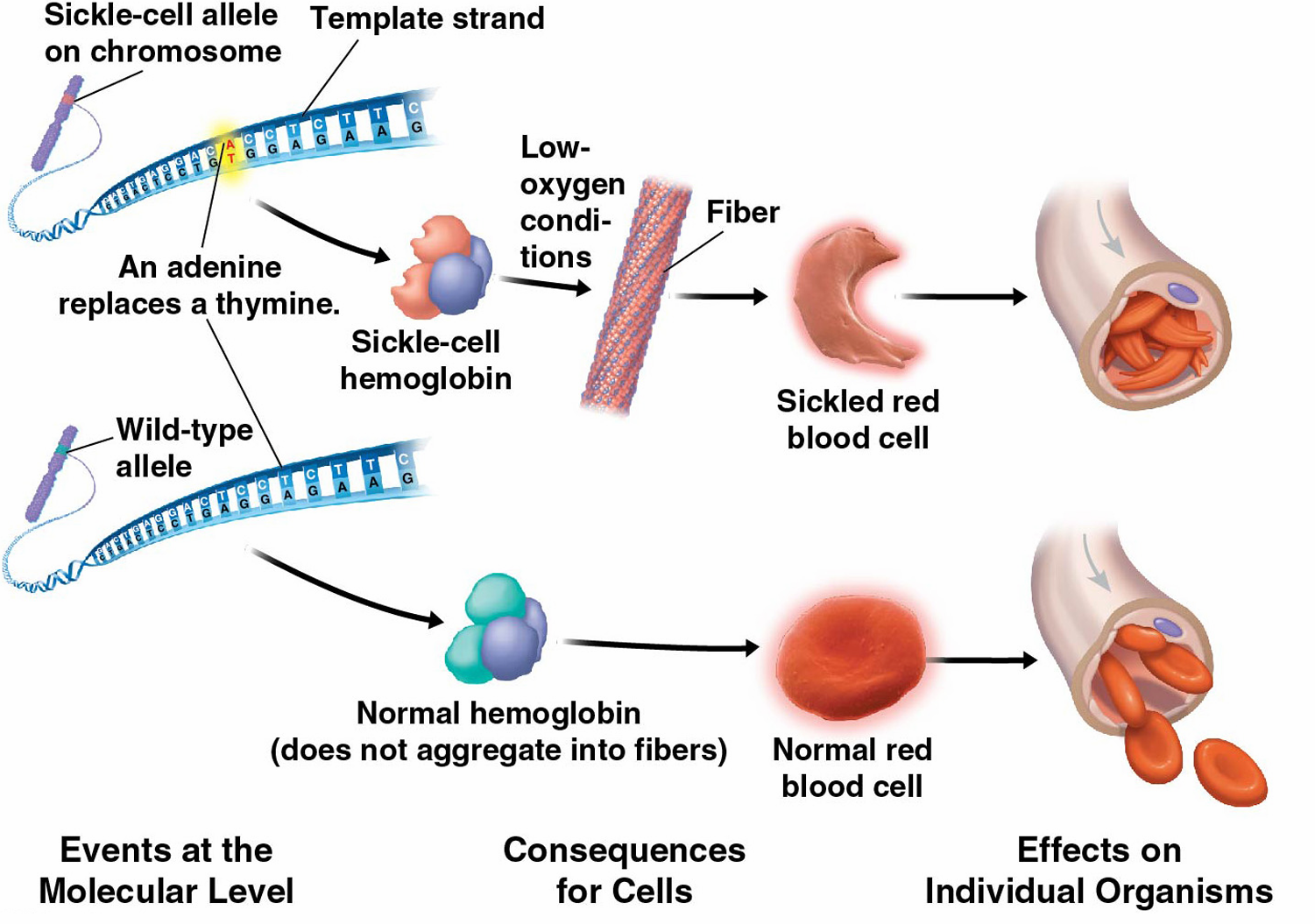
Point mutation in the gene for hemoglobin:
DNA change: A single base substitution (adenine replaced by thymine).
Protein effect: Glutamic acid is replaced by valine at position 6 in the β-globin chain.
This causes hemoglobin molecules to stick together, changing red blood cells into a sickle shape.
Consequences:
Reduced oxygen transport.
Blocked blood vessels, causing pain and tissue damage.
⚠️ Why Mutations Matter
- Even a single base change can drastically alter protein shape and function.
- Some mutations may be harmful, neutral, or rarely beneficial.
- Mutations contribute to genetic diversity but can also cause diseases.
Point mutations alter protein structure by changing amino acids; for example, sickle cell anemia results from a single amino acid substitution in hemoglobin.
Additional Higher Level
D1.2.12 – Directionality of Transcription and Translation
🔄 What is Directionality?
- DNA and RNA strands have directionality because their two ends differ chemically.
- One end is called 5′ (five prime) and the other 3′ (three prime).
- This direction matters because enzymes work only in one direction during transcription and translation.
🧬 Transcription Direction (5′ to 3′)
- During transcription, RNA polymerase adds RNA nucleotides only to the 3′ end of the growing RNA strand.
- This means the RNA strand grows from 5′ end towards 3′ end.
- The template DNA strand is read in the 3′ to 5′ direction to make RNA.
🧠 Translation Direction (5′ to 3′)
In translation, the ribosome moves along mRNA from the 5′ end towards the 3′ end.
The protein is built as the ribosome reads codons in this direction.
⚙️ Why Directionality Matters
Ensures accurate copying of genetic code during transcription.
Guarantees proper protein synthesis during translation.
Both transcription and translation proceed in the 5′ to 3′ direction, reflecting the chemical structure of nucleic acids and ensuring precise gene expression.
D1.2.13 – Initiation of Transcription at the Promoter
🔑 What is the Promoter?
The promoter is a specific DNA sequence located just before (upstream of) the gene to be transcribed.
It acts like a start signal for transcription.
🛠️ How Does Transcription Start?
- Special proteins called transcription factors bind to the promoter region.
- These factors help RNA polymerase attach to the DNA at the promoter.
- Once RNA polymerase is correctly positioned, it begins to unwind the DNA and starts synthesizing RNA.
⚙️ Key Points
- The promoter controls when and where a gene is transcribed.
- Transcription factors regulate gene expression by controlling RNA polymerase binding.
- Students don’t need to memorize the names of specific transcription factors, just their role.
Transcription starts at the promoter where transcription factors bind, enabling RNA polymerase to attach and begin making RNA from DNA.
D1.2.14 – Non-coding Sequences in DNA Do Not Code for Polypeptides
🧩 What Are Non-Coding Sequences?
Sections of DNA that do not code for proteins (polypeptides).
These sequences play important roles other than making proteins.
📌 Examples of Non-Coding DNA in Eukaryotes:
| Type | Function / Role |
|---|---|
| Regulators of Gene Expression | Control when and how much genes are turned on or off (e.g., promoters, enhancers). |
| Introns | Non-coding sections within genes that are removed during RNA processing. |
| Telomeres | Protective caps at the ends of chromosomes, preventing damage or fusion. |
| Genes for rRNAs and tRNAs | Code for RNA molecules essential for protein synthesis but not translated into proteins. |
⚠️ Key Notes:
Non-coding DNA is vital for gene regulation, chromosome stability, and protein production machinery.
They are not “junk DNA”; they have important functions beyond coding proteins.
Non-coding DNA sequences include gene regulators, introns, telomeres, and genes for rRNAs/tRNAs—all crucial for gene function and genome stability despite not coding for proteins.
D1.2.15 – Post-Transcriptional Modification in Eukaryotic Cells
🧬 What Is Post-Transcriptional Modification (PTM)?
PTM is the process where the initial RNA transcript (pre-mRNA) is chemically altered to form mature mRNA.
This is essential because only mature mRNA can be correctly translated into proteins in eukaryotic cells.
PTM increases stability and functionality of the mRNA.
🌿 Main Steps of Post-Transcriptional Modification
| Step | Process | Purpose |
|---|---|---|
| 1. Capping | Addition of a methylated guanine cap to the 5′ end of mRNA | Protects mRNA from degradation and helps ribosomes recognize the mRNA for translation |
| 2. Polyadenylation | Addition of a poly-A tail (many adenine nucleotides) to the 3′ end | Stabilizes mRNA and helps its export from nucleus to cytoplasm |
| 3. Splicing | Removal of introns (non-coding sequences) and joining of exons (coding sequences) | Produces a continuous coding sequence for protein synthesis |
🔬 Why Splicing Is Important
- Eukaryotic genes contain introns and exons.
- Introns do not code for proteins and must be removed.
- Splicing creates an accurate, continuous coding sequence.
- This process is carried out by a complex called the spliceosome.
📌 Other Types of Post-Transcriptional Modifications
| Modification | Description | Biological Role |
|---|---|---|
| N6-methyladenosine (m6A) | Most common PTM; methylation of adenosine bases in RNA | Regulates RNA stability, translation, and involved in diseases like cancer |
| Cleavage | Cutting RNA at specific sites | Helps process some RNAs for function |
| Thiolation | Addition of sulfur-containing groups | Affects RNA stability/function |
| Isopentenylation | Attachment of isopentenyl groups | Modifies RNA activity |
| Pseudouridine formation | Conversion of uridine to pseudouridine | Influences RNA folding and stability |
🌱 Dynamic and Reversible
PTM is not permanent.
Cells can reverse modifications to rapidly respond to environmental or developmental changes.
PTM transforms pre-mRNA into mature mRNA by adding a 5′ cap, a 3′ poly-A tail, and removing introns.
This process is essential for mRNA stability, nuclear export, and translation.
Other PTMs fine-tune RNA function and are important in gene regulation and health.
PTM is dynamic, allowing flexibility in gene expression.
D1.2.16 – Alternative Splicing of Exons to Produce Protein Variants
🧬 What Is Alternative Splicing?
- Alternative splicing is a post-transcriptional process where different combinations of exons are joined together from the same primary RNA transcript.
- This allows one gene to code for multiple different polypeptides (proteins).
- The primary transcript stays the same, but the mature mRNA varies depending on which exons are included or skipped.
🌿 How Does Alternative Splicing Work?
- The most common method is exon skipping, where certain exons are removed in some mRNA variants but retained in others.
- Other types include:
– Alternative 5’ or 3’ splice sites
– Mutually exclusive exons
– Intron retention (less common)
📌 Why Is Alternative Splicing Important?
- Increases protein diversity without increasing the number of genes.
- Produces tissue-specific or developmental-stage specific proteins.
- Helps organisms adapt and specialize functions of proteins in different cells or conditions.
🧠 Key Point
Alternative splicing means one gene → many protein variants.
This expands the proteome (all proteins an organism can make) without needing more genes.
Alternative splicing allows flexible gene expression by combining exons in different ways.
This process dramatically increases the variety of proteins from a single gene.
It is crucial for complexity and specialization in eukaryotic organisms.
D1.2.17 – Initiation of Translation
🧬 What Is Initiation of Translation?
The first step in making proteins where the ribosome assembles on the mRNA and gets ready to build the polypeptide chain.
Happens in the cytoplasm of eukaryotic cells.
🌿 Steps of Initiation
- Small ribosomal subunit (40S in eukaryotes) attaches to the 5′ end of the mRNA.
- It scans along the mRNA to find the start codon (AUG).
- The initiator tRNA carrying methionine pairs its anticodon with the start codon at the P site of the ribosome.
- The large ribosomal subunit (60S) joins to form the complete 80S ribosome.
- A second tRNA carrying an amino acid bind to the A site, ready to add the next amino acid.
🔬 Roles of Ribosome Binding Sites
| Site | Function |
|---|---|
| A site (Aminoacyl site) | Holds the tRNA with the next amino acid to be added |
| P site (Peptidyl site) | Holds the tRNA carrying the growing polypeptide chain (starts with initiator tRNA) |
| E site (Exit site) | Where empty tRNA leaves the ribosome after its amino acid is added |
📌 Additional Details
- In eukaryotes, initiation factors like eIF1 and eIF1A help the small subunit recognize the start codon and position the initiator tRNA correctly.
- The process requires energy from GTP to assemble the ribosome and place tRNAs.
- The small subunit scans from the 5′ cap of mRNA to locate the start codon before large subunit binds.
Initiation sets up the ribosome on mRNA with the initiator tRNA at the start codon in the P site.
The small subunit binds first and scans for the start codon.
The large subunit joins to complete the ribosome.
Ribosome has three tRNA sites (A, P, E) crucial for translation elongation.
D1.2.18 – Modification of Polypeptides into Their Functional State
🧬 What Is Post-Translational Modification?
After translation, many polypeptides are inactive and require modification to become functional proteins.
These modifications are called post-translational modifications (PTMs).
🌿 Types of Modifications
| Modification | Description | Purpose / Effect |
|---|---|---|
| Cleavage | Cutting parts of the polypeptide chain | Activates protein by removing unnecessary segments (e.g., C-peptide removal) |
| Phosphorylation | Addition of phosphate groups | Alters activity or function, important in regulation |
| Disulfide bridge formation | Bonds form between cysteine amino acids | Stabilizes 3D protein structure |
| Glycosylation | Attachment of sugar groups | Improves stability and targeting of proteins |
| Removal of amino acids | Sometimes first methionine or others are removed | Finalizes active protein structure |
| Conjugation with cofactors | Addition of non-protein groups | Essential for protein function |
🔬 Example: Modification of Insulin
- Insulin is produced as pre-proinsulin (inactive precursor).
- It is first processed to proinsulin by removal of the signal peptide.
- Then, C-peptide is cleaved off from proinsulin to form active insulin.
- This two-step cleavage is essential for insulin’s correct function.
🧠 Protein Folding Assistance
Chaperone proteins like Hsp70 and Hsp60 help newly made polypeptides fold correctly.
Proper folding is crucial for protein activity and stability.
Polypeptides often need chemical or structural changes to become active proteins.
These include cleavage, phosphorylation, glycosylation, and disulfide bonds.
Insulin is a classic example: made as pre-proinsulin, processed to proinsulin, then to active insulin.
Molecular chaperones assist in folding and stabilization during this process.
D1.2.19 – Recycling of Amino Acids by Proteasomes
🧬 What Are Proteasomes?

Proteasomes are protein complexes that break down unwanted or damaged proteins inside cells.
This process is called proteolysis and results in recycling amino acids.
🌿 How Proteasomes Work
- Proteins are marked for destruction by chemical tags added by specific enzymes.
- The proteasome breaks peptide bonds, cutting proteins into amino acids.
- These amino acids are recycled and reused to build new proteins.
📌 Why Is Protein Recycling Important?
- Keeps the cell’s protein content (proteome) functional and balanced.
- Controls protein levels, regulating many cellular processes.
- Prevents accumulation of damaged or faulty proteins.
Maintaining a functional proteome requires constant protein breakdown and synthesis.
Proteasomes play a vital role by recycling amino acids from degraded proteins for reuse.
