IB DP Biology D2.3 Water potential Study Notes - New Syllabus -2025
IB DP Biology D2.3 Water potential Study Notess – New syllabus 2025
IB DP Biology D2.3 Water potential Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on IB Biology syllabus with guiding questions of
- What factors affect the movement of water into or out of cells?
- How do plant and animal cells differ in their regulation of water movement?
Standard level and higher level: 2 hours
Additional higher level: 2 hours
D2.3.1 – Solvation with Water as the Solvent
🧠 What is Solvation?
- Solvation is when a solvent (like water) surrounds and interacts with solute molecules or ions.
- Water’s ability to dissolve substances depends on its polarity:
- Oxygen atom has a partial negative charge (δ⁻)
- Hydrogen atoms have a partial positive charge (δ⁺)
🌿 How Water Solvates Solutes
- Polar molecules dissolve because their charges attract the partial charges on water:
- Positive parts of solutes attract water’s oxygen (δ⁻)
- Negative parts attract water’s hydrogen (δ⁺)
- Ions (charged particles) are attracted to opposite charges on water:
- Cations (positive ions) attract water’s oxygen atoms
- Anions (negative ions) attract water’s hydrogen atoms
🔬 Hydration Shells
- Water molecules form “shells” around ions or charged molecules, preventing them from clumping (precipitating).
- This keeps ions dissolved and free to move, important for cellular processes.
- Example: When sodium chloride (NaCl) dissolves, ionic bonds break, and water molecules surround Na⁺ and Cl⁻ ions.
📌 Why Is This Important?
- Cytoplasm is a complex solution of many dissolved substances.
- Water’s solvation allows metabolic reactions to occur efficiently.
Water dissolves substances by forming hydrogen bonds and electrostatic attractions.
Positively charged ions attract water’s oxygen; negatively charged ions attract water’s hydrogens.
Water molecules surround solutes, creating hydration shells to keep them dissolved.
D2.3.2 – Water Movement: From Less to More Concentrated Solutions
🧠 Key Idea: Direction of Water Movement
- Water moves from a solution with lower solute concentration to one with higher solute concentration.
- Movement is driven by differences in solute concentration, NOT water concentration.
- This net movement is called osmosis.
🌿 Important Terms
| Term | Meaning |
|---|---|
| Hypotonic | Solution with lower concentration of osmotically active solutes |
| Hypertonic | Solution with higher concentration of osmotically active solutes |
| Isotonic | Solutions with equal concentrations of osmotically active solutes |
🔬 How Osmosis Works
- Water molecules constantly move both ways across a membrane.
- However, more water moves from the hypotonic (less concentrated) solution to the hypertonic (more concentrated) one.
- This results in a net movement of water toward the higher solute concentration.
- When two solutions are isotonic, water moves equally both ways → no net movement (dynamic equilibrium).
🌿 Why Does This Happen?
- Water molecules are attracted to solute particles (like sodium, potassium, chloride ions, glucose).
- These solute-water attractions reduce free water molecules available to move.
- So, water tends to move towards the side with more solutes to balance concentrations.
Water moves from hypotonic to hypertonic solutions by osmosis.
Osmosis depends on differences in solute concentration, not water concentration.
Isotonic solutions have equal solute concentrations → no net water movement.
Solutes must be osmotically active (able to attract water) for osmosis to occur.
D2.3.3 – Water Movement by Osmosis Into or Out of Cells
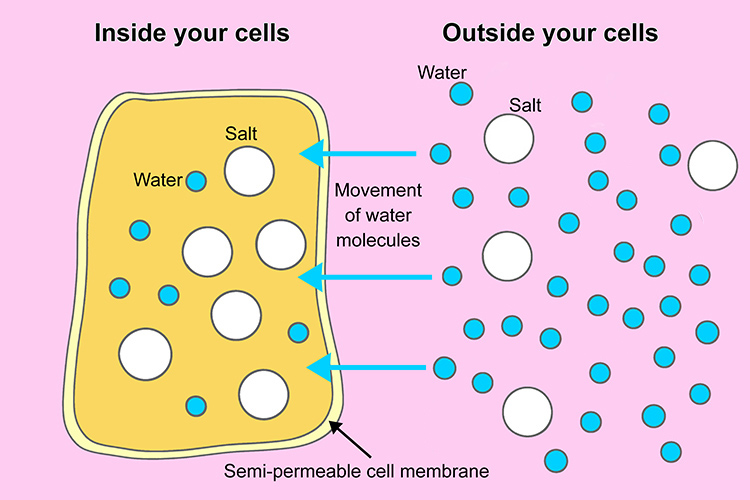
🧠 How Water Moves Across Cell Membranes
- Cells have a plasma membrane separating their cytoplasm from outside fluids.
- The membrane is highly permeable to water, but less permeable to solutes.
- When solute concentrations differ inside and outside the cell, water moves across the membrane, not solutes.
- This water movement is called osmosis.
🌿 Osmosis Direction Depends on Solute Concentration
| Environment Outside Cell | Direction of Water Movement | Effect on Cell |
|---|---|---|
| Hypotonic (lower solute) | Water moves into the cell | Cell may swell or burst |
| Hypertonic (higher solute) | Water moves out of the cell | Cell may shrink or shrivel |
| Isotonic (equal solute) | Water moves both ways equally (dynamic equilibrium) | Cell volume stays stable |
🔬 Important Points
- Osmosis is a passive process; it requires no energy.
- Cells can regulate osmosis by changing their plasma membrane’s permeability to water.
- Cells can also control osmosis by adjusting the concentration of solutes inside (making cytoplasm more or less hypertonic).
- Example: Root cells absorb water from soil because their cytoplasm is hypertonic relative to soil water.
Water moves across the plasma membrane from hypotonic to hypertonic solution by osmosis.
Cells swell in hypotonic environments and shrink in hypertonic ones.
In isotonic conditions, water moves equally both ways → dynamic equilibrium.
Cells can regulate water movement by changing solute concentration or membrane permeability.
D2.3.4 – Changes in Plant Tissue Due to Water Movement in Hypotonic and Hypertonic Solutions
🧠 What Happens When Plant Tissue Is Bathed in Different Solutions?
- When plant tissue is placed in a hypotonic solution (lower solute concentration outside):
- Water moves into the tissue by osmosis.
- Tissue gains mass and length (swells).
- When bathed in a hypertonic solution (higher solute concentration outside):
- Water moves out of the tissue.
- Tissue loses mass and length (shrinks).
🌿 Experiment to Measure Changes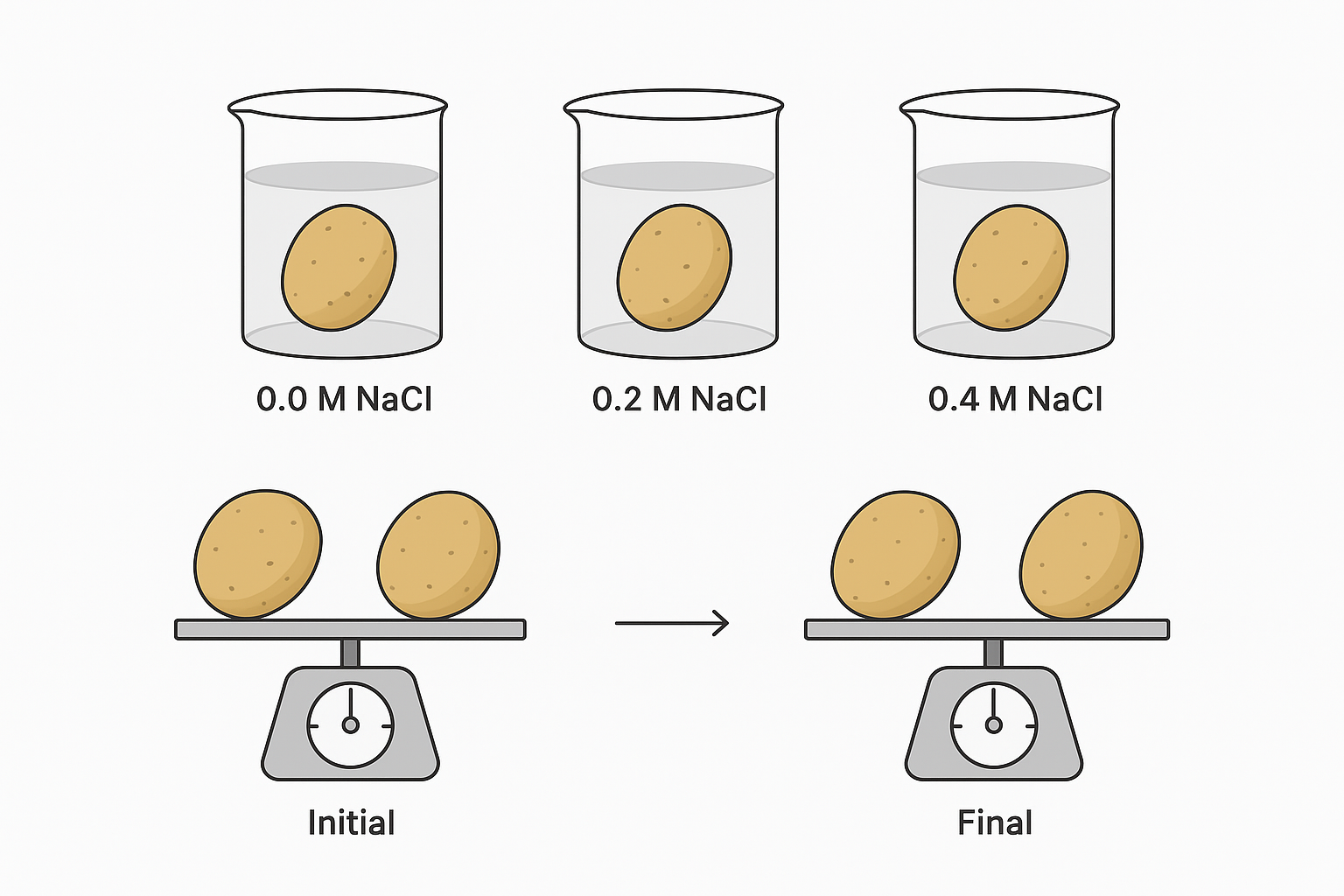
- Use homogeneous plant tissue like potato tubers.
- Prepare a range of sodium chloride (NaCl) solutions of different concentrations.
- Steps to ensure accuracy:
- Use similar-sized tissue samples.
- Dry samples before weighing at start and end.
- Keep all variables constant except solute concentration.
- Leave tissue in solutions long enough to see change, but not too long (avoid decay).
🔍 Data Analysis
- Measure changes in mass and length of tissue samples.
- Plot results to find the isotonic concentration where tissue mass/length doesn’t change.
- Use standard deviation and standard error from repeated trials to:
- Assess reliability of measurements.
- Show error bars on graphs.
Water movement causes plant tissue to swell in hypotonic solutions and shrink in hypertonic solutions.
Measuring mass and length changes helps find the isotonic point.
Statistical tools like standard deviation and standard error improve data reliability.
D2.3.5 – Effects of Water Movement on Cells That Lack a Cell Wall
🧠 Key Difference: Cells Without Cell Walls
| Property | Plasma Membrane (Animal & Plant Cells) | Cell Wall (Plants Only) |
|---|---|---|
| Main Constituent | Phospholipids | Cellulose |
| Thickness | Thin (~5 nm or less) | Thick (250 nm to 5 μm or more) |
| State | Liquid — flexible, allows vesicle formation | Solid — rigid, molecules do not diffuse |
| Tensile Strength | Very low, easily torn | Very high — stronger than steel |
| Permeability | Semi-permeable – some solutes cannot pass freely | Freely permeable unless waterproofed |
🌿 Water Movement in Cells Without Cell Walls
- In hypotonic solutions (lower solute outside):
- Water moves into the cell by osmosis.
- Cell swells because it cannot resist pressure without a cell wall.
- Eventually, the cell bursts (lysis) due to lack of support.
- Example: Red blood cells burst in pure water → form “red cell ghosts.”
- In hypertonic solutions (higher solute outside):
- Water moves out of the cell.
- Cell shrinks and wrinkles — called crenation in animal cells.
🔬 Special Cases
- Freshwater unicellular organisms (e.g., Amoeba proteus):
- Lack cell walls.
- Constantly take in water by osmosis.
- Use contractile vacuoles to pump excess water out and prevent bursting.
- Multicellular organisms:
- Must maintain isotonic tissue fluid (same solute concentration as cells).
- Prevents harmful swelling or shrinkage of cells.
Cells without walls rely on flexible membranes, which cannot resist swelling pressure.
Hypotonic environments cause swelling and bursting; hypertonic environments cause shrinkage.
Contractile vacuoles help freshwater unicellular organisms remove excess water.
Multicellular organisms maintain isotonic fluids to keep cells healthy.
D2.3.6 – Effects of Water Movement on Cells With a Cell Wall
🧠 Water Movement in Plant Cells
Plant cells have a strong cell wall surrounding the plasma membrane.
The cell wall prevents bursting when water enters by osmosis.
🌿 Turgor Pressure in Hypotonic Solutions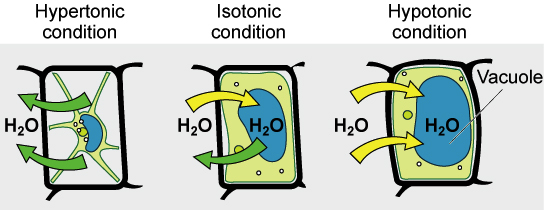
- When plant cells are in a hypotonic environment (lower solute outside), water moves into the cell.
- Water fills the cytoplasm and pushes the plasma membrane against the cell wall.
- This pressure is called turgor pressure.
- The cell becomes turgid (swollen) – a healthy state.
- Turgid cells help support stems and leaves, allowing plants to stand upright.
🌿 Effects of Water Loss in Hypertonic Solutions
- In a hypertonic environment (higher solute outside), water moves out of the cell.
- Cytoplasm volume decreases and pressure drops.
- The plasma membrane pulls away from the cell wall, causing plasmolysis.
- The cell becomes flaccid (limp).
- Wilting occurs in plants when many cells lose water.
- Plasmolysis is damaging and can lead to cell death.
- Happens naturally when plants are exposed to salty conditions like seawater flooding.
Plant cell walls provide strength to resist bursting in hypotonic solutions.
Turgor pressure from water uptake keeps cells firm and supports plants.
Loss of water in hypertonic solutions causes plasmolysis, damaging cells.
Flaccid cells lead to wilting, a sign of water stress in plants.
D2.3.7 – Medical Applications of Isotonic Solutions
🧠 Why Use Isotonic Solutions Medically?
- Hypertonic solutions can dehydrate human cells, causing damage.
- Hypotonic solutions can make cells swell and burst.
- Isotonic solutions keep cells healthy because water moves in and out equally.
- This balance is crucial during medical treatments to avoid harming cells.
🌿 Normal Saline: The Common Isotonic Solution
- Normal saline is 0.9% sodium chloride (NaCl) solution.
- Contains 9 g NaCl per liter (~0.154 mol dm⁻³).
- Matches the solute concentration of body fluids, so it is safe for cells.
🔬 Medical Uses of Normal Saline
| Application | Purpose |
|---|---|
| Intravenous (IV) drip | Rehydrates patients, restores fluid balance in blood |
| Wound and skin irrigation | Cleans wounds to prevent infection |
| Moistening damaged skin before grafts | Keeps tissue healthy and prevents drying out |
| Eye drops | Provides safe moisture to eyes without irritation |
| Organ preservation before transplantation | Frozen saline slush cools hearts, kidneys, and other organs |
Isotonic solutions prevent cell damage by maintaining water balance.
Normal saline is widely used in hospitals for rehydration and cleaning.
It protects cells during organ transplants and skin treatments.
Additional Higher Level
D2.3.8 – Water Potential as the Potential Energy of Water per Unit Volume
🧠 What Is Water Potential?
- Water potential (Ψ) measures the potential energy of water per unit volume.
- It predicts the direction water will move in living systems, especially plants.
- Symbol: Ψ (Greek letter psi).
- Units: kilopascals (kPa) or megapascals (MPa).
- Absolute water potential can’t be measured, so values are relative.
- Pure water at atmospheric pressure and 20°C is set as zero water potential (Ψ = 0).
🌿 Factors Affecting Water Potential
| Factor | Effect on Water Potential |
|---|---|
| Hydrostatic Pressure | ↑ Pressure → ↑ Water potential (more positive) |
| Solute Concentration | ↑ Solutes → ↓ Water potential (more negative) due to reduced free water molecules |
🔬 How Water Potential Works
- Water moves from areas of higher water potential to areas of lower water potential.
- Pure water (Ψ = 0) has the highest possible water potential.
- Adding solutes lowers water potential because solutes bind water molecules, reducing their energy.
- Pressure (like turgor pressure) can increase water potential by pushing water.
Water potential predicts water movement direction.
Measured relative to pure water at standard conditions (Ψ = 0).
Higher pressure raises water potential; more solutes lower it.
Water moves from high Ψ → low Ψ areas in plants and cells.
D2.3.9 – Movement of Water from Higher to Lower Water Potential
🧠 Why Does Water Move?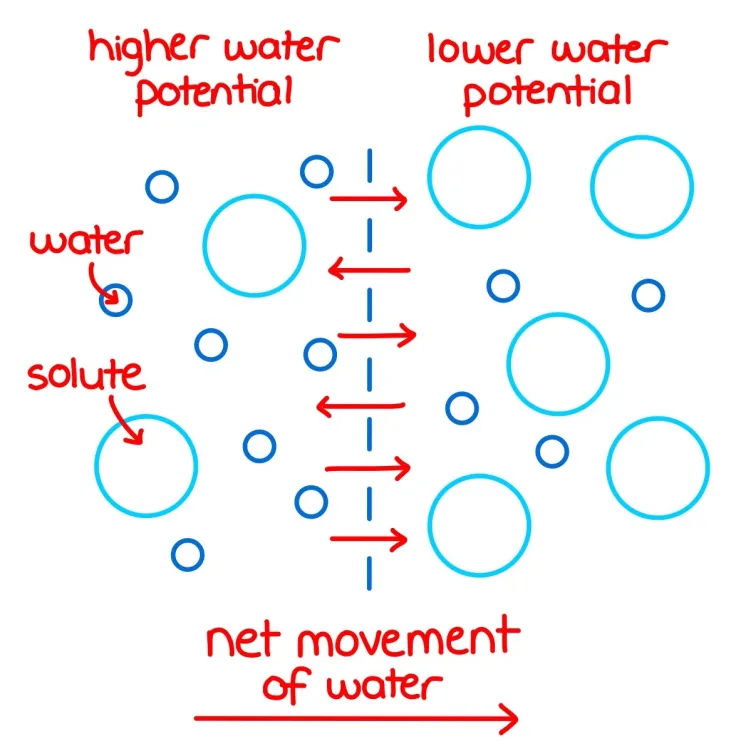
- Water moves from an area of higher water potential to an area of lower water potential.
- This movement happens to minimize potential energy, making the system more stable.
- Similar examples:
- Rocks roll downhill due to gravity (move to lower potential energy).
- Atoms form chemical bonds to lower their energy state.
🌿 Water Potential in Cells
- Pure water has the highest water potential: 0 kPa.
- Cells contain solutes, so their water potential is negative (e.g., −200 kPa).
- Water moves from a cell or solution with a less negative water potential to one with a more negative water potential.
- For example, water moves from −200 kPa to −300 kPa.
Water movement reduces potential energy in biological systems.
Water flows from high Ψ (less negative) to low Ψ (more negative).
This principle explains water uptake in plants and cells.
D2.3.10 – Contributions of Solute Potential and Pressure Potential to Water Potential in Cells With Walls
🧠 Water Potential Equation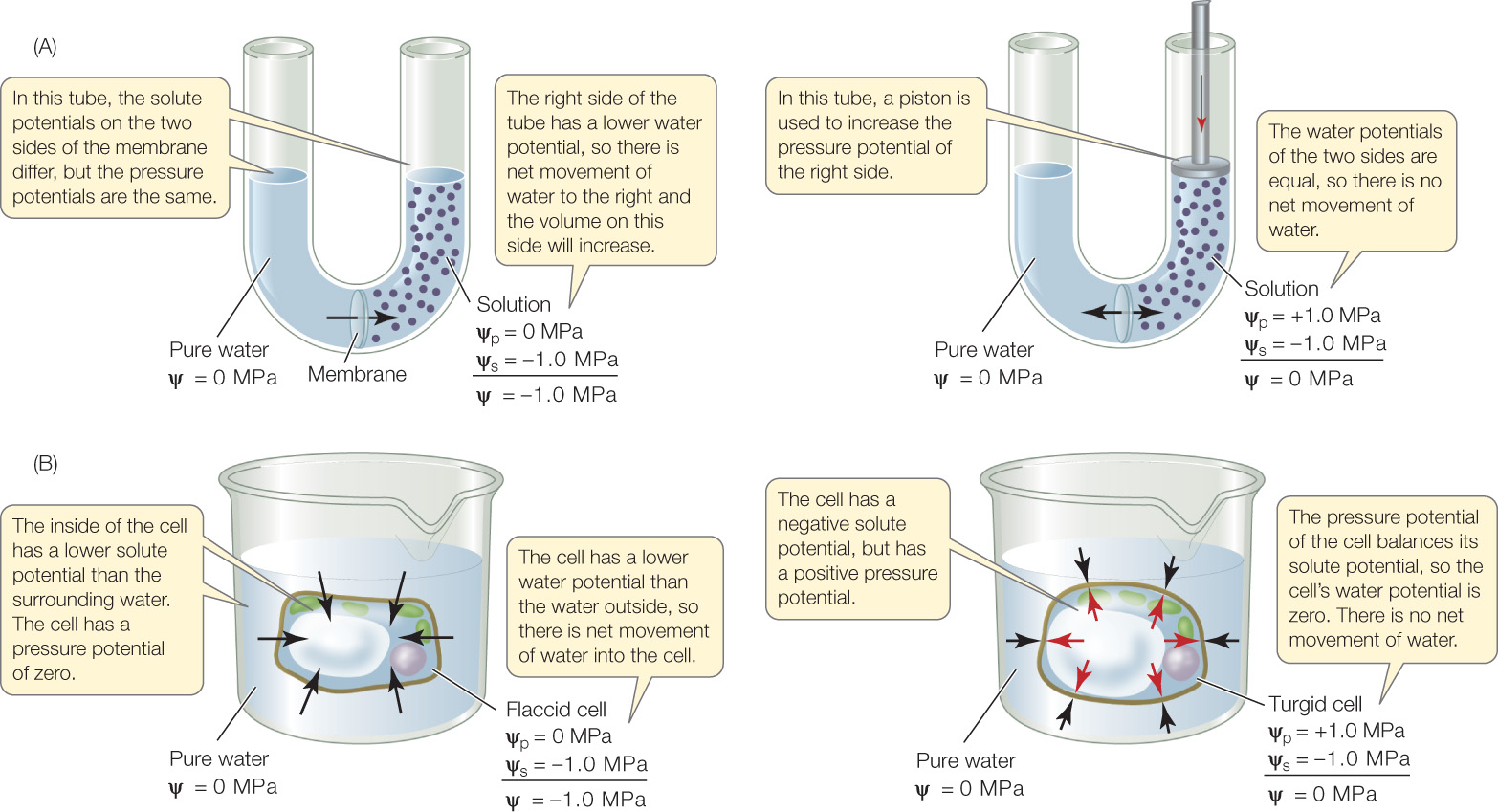
Ψw = Ψs + Ψp
Where:
- Ψw = Water potential
- Ψs = Solute potential (osmotic potential)
- Ψp = Pressure potential
🌿 Solute Potential (Ψs)
- Also called osmotic potential.
- Adding solutes makes Ψs more negative (lowers water potential).
- Pure water has Ψs = 0 (no solutes).
- More solutes → lower (more negative) Ψs.
- Solutes reduce free water molecules, decreasing potential energy.
🌿 Pressure Potential (Ψp)
- Pressure inside cells affects water potential.
- Positive pressure (above atmospheric) → increases water potential (Ψp > 0).
- Example: Turgor pressure in healthy plant cells.
- Negative pressure (below atmospheric) → decreases water potential (Ψp < 0).
- Example: Tension in xylem vessels pulling water upward.
- At atmospheric pressure, Ψp = 0.
🔍 Key Concepts
- Water potential depends on both solute concentration and pressure.
- Solutes always lower water potential (make it negative).
- Pressure can either raise or lower water potential depending on its sign.
- Water moves from regions of higher Ψw to lower Ψw.
| Component | Effect on Water Potential | Typical Values |
|---|---|---|
| Solute Potential (Ψs) | Lowers water potential (more negative) | ≤ 0 (zero for pure water) |
| Pressure Potential (Ψp) | Raises or lowers water potential depending on pressure | Usually positive in cells, negative in xylem tension |
Water potential is determined by solute potential and pressure potential.
Solutes always lower water potential (make it more negative).
Pressure potential can raise or lower water potential depending on its sign.
Water moves from areas of higher to lower water potential.
D2.3.11 – Water Potential and Water Movements in Plant Tissue
🌿 Bathing Plant Tissue in Hypotonic Solutions
- Hypotonic solutions have higher water potential (Ψ) than plant cells.
- Water moves into the cells by osmosis.
- Inside the cell:
- High solute concentration lowers solute potential (Ψs).
- This decreases overall water potential, encouraging water uptake.
- Water intake increases pressure potential (Ψp) → turgor pressure.
- Cells become turgid (swollen and firm).
- When internal water potential equals external, water movement stops.
- In pure water (Ψ = 0), cells reach maximum turgor pressure, preventing bursting.
🌿 Bathing Plant Tissue in Hypertonic Solutions
- Hypertonic solutions have lower water potential than plant cells.
- Water moves out of the cells.
- Initially, cells have positive pressure potential (Ψp) due to turgor.
- As water leaves:
- Pressure potential decreases.
- Cells become flaccid (limp).
- Continued water loss causes plasmolysis: plasma membrane pulls away from the cell wall.
- At extreme dehydration, internal water potential matches external, stopping water movement.
| Condition | Water Potential Outside | Water Movement | Cell State | Key Effects |
|---|---|---|---|---|
| Hypotonic | Higher (less negative) | Into cells | Turgid | Cells swell, pressure builds up |
| Hypertonic | Lower (more negative) | Out of cells | Flaccid → Plasmolysed | Cells shrink, membrane detaches |
