13.1 First-row d-block elements
Essential Idea:
The transition elements have characteristic properties; these properties are related to their all having incomplete d sublevels.
Understandings:
- Transition elements have variable oxidation states, form complex ions with ligands, have coloured compounds, and display catalytic and magnetic properties.
- Zn is not considered to be a transition element as it does not form ions with incomplete d-orbitals.
- Transition elements show an oxidation state of +2 when the s-electrons are removed.
Applications and Skills:
- Explanation of the ability of transition metals to form variable oxidation states from successive ionization energies.
- Explanation of the nature of the coordinate bond within a complex ion.
- Deduction of the total charge given the formula of the ion and ligands present.
- Explanation of the magnetic properties in transition metals in terms of unpaired electrons.
13.1 First-row d-block elements – Facts
The transition elements have characteristic properties; these properties are related to their all having incomplete d sub-levels.
• Transition metals have partially filled d orbitals in their atoms or ions.
• Zn is not a transition element because it has a full d sub-level in its atoms and ions.
• Characteristic properties include: variable oxidation number, complex ion formation with ligands, existence of coloured compounds, have catalytic and magnetic properties.
• Multiple oxidation states arise because the 3d and 4s sub-levels are close in energy and both are involved in bonding.
• All d-block elements except Sc show an oxidation state of +2.
• All d-block elements except Zn show an oxidation state of +3.
• A ligand is a molecule or negative ion that donates a pair of electrons to a central metal ion to form a covalent (coordinate) bond. They are Lewis bases.
• Complex ions are formed when a central metal ion is bonded to a ligand with a coordinate bond. Examples include [Fe(H2O)6]3+, [Fe(CN)6]3–, [CuCl4]2–, and [Ag(NH3)2]+.
• The charge on a complex ion is the sum of the charges of the metal ion and the ligands.
• Transition metals act as heterogeneous catalysts as they can provide a surface for reaction: they use the 3d and 4s electrons to form weak bonds to reactant molecules.
• Magnetic properties are a result of unpaired electrons in the transition metal atom or ion.
Electronic configuration
The first-row d-block contains 10 elements because the 3d sub-level has 5 orbitals each accommodating two electrons. These 10 elements, although they are in the same row and therefore in different groups, show a lot of similarities but also some changes. However, as you move across the row these changes are only very gradual (=transitional) as opposed to the more pronounced changes when you go across periods 2 and 3.
The reasons why these elements share properties so closely must be found in their electronic structures and the relative energy levels within their atoms.
The following are characteristic properties of transition elements:
variable oxidation states | complex ion formation | coloured compounds | catalytic properties | magnetic properties |
To be able to explain these properties, we need to know the electronic configuration of these first row transition metals. Complete the electronic configurations below using the Aufbau rules of the first 10 transition elements.
Answer/Explanation
Ans
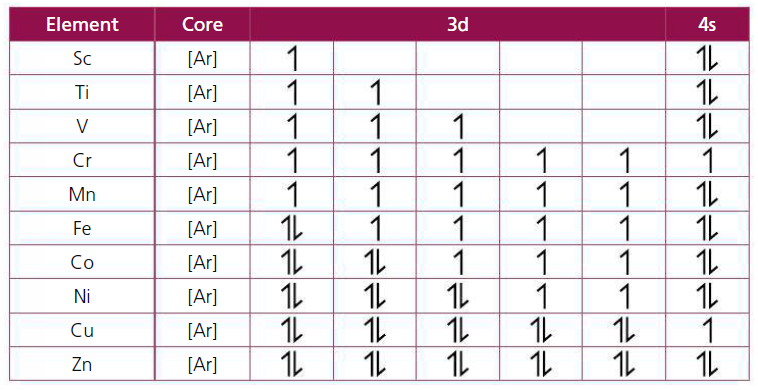
Exceptions to the general patterns in the electron arrangement are:
* Cr: [Ar] 3d5 4s1 instead of [Ar] 3d4 4s2 as the first is the preferred arrangement and appears to be more stable in terms of energy; the 3d sub-level is half-filled (a half-filled or filled or empty orbital has greater stability than a partially filled orbital although this does not always apply) which gives a lower total energy; because the 3d and 4s are so close together they can be considered as degenerate orbitals. This 3d54s1 only happens in the electronic configuration of Cr atoms * Cu: [Ar] 3d10 4s1 instead of [Ar] 3d9 4s2 because filling up of 3d is a more stable arrangement. |
Electronic configuration of transition metal ions
You recall that when filling up orbitals, the 4s orbital is filled before the 3d orbitals. This is because in the empty atom, the 4s orbital has a lower energy than 3d orbitals. However, once the electrons are actually in the 4s orbitals, the energy order changes; the 4s electrons repel each other and this increases the 4s energy level beyond the 3d energy level. When the 3d sublevel is being filled it is at an energy level below 4s.
As a result in all the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital when it has electrons in it. So the reversed order of the 3d and 4s orbitals only applies to building the atom up in the first place. In all other respects, you treat the 4s electrons as being the outer electrons.
As the 3d electrons are on a slightly lower energy level than the 4s electrons when the transition metal atoms ionise, it are the 4s electrons (they are also shielded from the nuclear charge by the 3d electrons) which are removed first before the 3d electrons.
Complete the table below
species | electronic configuration | species | electronic configuration |
Sc3+ |
| Fe2+ |
|
Ti4+ |
| Fe3+ |
|
V5+ |
| Co2+ |
|
Cr3+ |
| Ni2+ |
|
Cr6+ |
| Cu+ |
|
Mn2+ |
| Cu2+ |
|
Mn3+ |
| Zn2+ |
|
Variable oxidation states (except zinc). Why? Similar successive ionization energies of 3d and 4s electrons
- Oxidation state of a transition element in a compound or molecular ion = number of electrons released/sharing in either covalent (sometimes the bonds formed have a greater covalent character) or ionic bonds.
- When writing an oxidation state we write the sign first then the number e.g. +3 unlike an ionic charge which as number first and then the sign e.g. 3+.
- All transition elements, except Sc (+3 only) and Zn (+2 only), have more than one stable oxidation state.
Common oxidation state: as first and second ionization energies are very similar in all transition elements, all first row d-block elements have + 2 as an oxidation state which corresponds with the loss of the 4s electrons. Exceptions are Cr and Cu that lose the single 4s electron and one 3d electron to have +2 as their oxidation state. Similar trends occur in other rows of transition metals
- You should be familiar with the following oxidation states (in brackets are their configurations):

- Transition metals have variable oxidation states because the 3d and 4s electrons have similar successive ionization energies (only a gradual increase) because the five inner d orbitals are at a similar energy level as the single 4s orbital.
- The only gradual increase in successive ionization energies is because there is less repulsion every time an electron is removed so electrons are attracted more strongly by the nucleus. However, the decrease in repulsion is also only gradual.
One first row d-block element is not considered a transition element
The definition below is a recent development as the part about an atom has been added which now means that scandium is considered a transition element with its [Ar] 4s23d1 which it was not before this change in the definition.
A transition element is a d-block element that has an atom with a partially filled d-sub-level or that forms at least one stable cation that has a partially filled d-sub-level.
As Zn does not have neither an atom or a stable ion, Zn2+, with a partially-filled d-sub-level Zn is not considered a transition metal. Zinc also does not form any coloured compounds, only has one oxidation state and has no catalytic activity. Many of these properties also fit scandium but this is now considered a transition metal a it has an atom with a partially-filled d sub-level.
Formation of complexes or complex ions (result of the high charge density of the metal ion)
A complex or complex ion has a metal ion at its centre around which there are a number of other molecules or negative ions. A complex is a compound that is formed when a ligand, that has been attracted by the charge of the transition metal ion, donates an electron pair (= dative bond) into an empty low energy orbital (3d, 4s or 4p) of the metal ion.
These complexes/complex ions are usually formed when transition metals are dissolved in water; or become hydrated. However, these complexes also form in other circumstances.
When the complex is charged it is called a complex ion; the charge on the ion is delocalised over the entire complex as indicated by the square brackets.
Transition metals can form complexes because their ions have a high charge density:
- they have quite a large nuclear charge but are relatively small;
- the 3d electrons are not so effective (as 2s or 2p electrons) at shielding the effect of the ionic charge which really comes from the nucleus.
Molecules like water, ammonia and negative molecular ions can all act as ligands as they have at least one lone pair.
A ligand = a molecule or negative ion which contains a non-bonding electron pair which it uses to form a dative bond with the central ion in a complex.
Different types of ligands
Ligands can be classified according to their number of atoms that can donate non-bonding pairs and therefore the number of coordinate bonds that they can form:
- monodentate ligands can only form 1 coordinate bond as they have only one atom that can donate non-bonding pair/form coordinate bond. Example are water (only oxygen atom can), ammonia and any halide ion.
- polydentate ligands can form 2 or more coordinate bonds as they have 2 or more atoms that can donate non-bonding pairs e.g. EDTA, 1,2-ethanediamine as both molecules have 2 nitrogen atoms in them. EDTA can make 6 coordinate bonds so one EDTA molecule can make a complex ion with an iron (II) or iron (III) ion.
Co-ordination number
The number of ligands that are attached to such a metal ion is referred to as the co-ordination number.
Common co-ordination numbers are:
Cu = 4 Fe = 6 Ag = 2
Examples of common complex ions
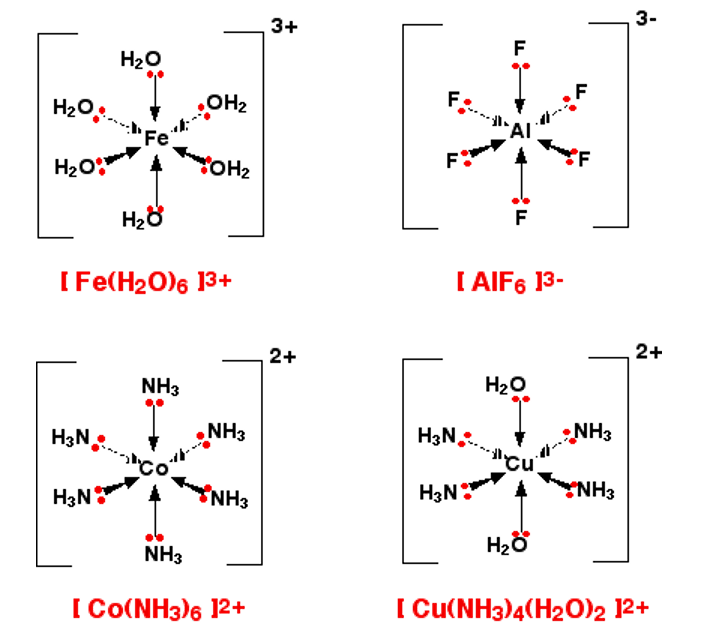
[Fe(H2O)6]3+ [Fe(CN)6]3- [CuCl4]2- [Cu(NH3)4]2+ [Ag(NH3)2]+
Shapes of complex ions
Complexes also have shapes which we can predict using the VSEPR theory. The shape depends on the number of ligands:
- if co-ordination number = 6 then shape = octahedral
- if co-ordination number = 4 then shape = tetrahedral (or square planar = less common)
- if co-ordination number = 2 then shape = linear
Examples of octahedral ions
Magnetic properties
In terms of magnetic properties (attracted or repelled by an external magnetic field) there are 3 types of materials:
- Paramagnetic materials are magnetic i.e. they are attracted weakly to a magnetic field.
In the case of the transition metals, atoms or ions that contain unpaired d electrons behave like small magnets. Because of their spin electrons create a magnetic field that can align itself to an external electric or magnetic field when exposed to it; unpaired electrons can do this because they can spin in any direction – they create a net magnetic moment. As a result atoms with unpaired d-electrons are weakly attracted to a magnet. The greater the number of unpaired electrons the more paramagnetic the material. The alignment is only temporary so they are only magnetic for a short period of time.
Examples of paramagnetic materials in the first row d-block:
- atoms: all apart from Zn.
- ions e.g. in compounds and complexes: e.g. Mg2+ has 5 unpaired electrons, Co2+ (3 unpaired
electrons), Ni2+ (2 unpaired electrons), Fe2+ (unpaired electrons).
- Diamagnetic materials only have paired d-electrons and therefore create a magnetic field opposed or not aligned to an external field that is why they are weakly repelled by that external field. The paired electrons cancel out each other’s magnetic field so there is no net magnetic moment in the atom or ion.
Examples of diamagnetic materials: Zn and Zn2+.
- Ferromagnetic materials can be made into permanent magnets as their electron alignment caused by an external magnet can be made permanent e.g. by heating and cooling it in a magnetic field.
Examples of ferromagnetic materials are iron, cobalt and nickel.
