IBDP Physics- B.4 Thermodynamics- IB Style Questions For HL Paper 2 -FA 2025
Question
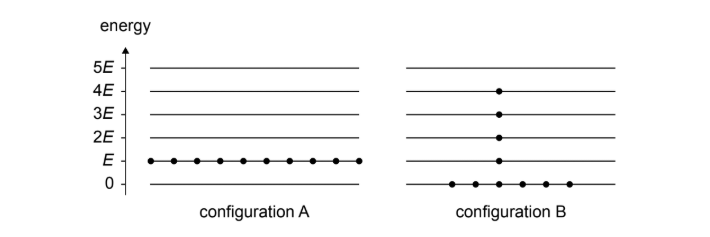
Process 1: the system starts in configuration A and changes to configuration B.
Process 2: the system starts in configuration B and changes to configuration A.
(b) Explain why process 1 is more likely to take place.
Most-appropriate topic codes (IB Physics):
▶️ Answer/Explanation
(a)
Configuration B has the greater number of microstates. In configuration A all particles have exactly the same energy \(E\), so there is only one possible microscopic arrangement. In configuration B, the total energy can be distributed among the particles in many different ways while giving the same overall appearance, resulting in a much larger number of microstates.
(b)
Process 1 is more likely because, for an isolated system, the entropy tends to increase in accordance with the second law of thermodynamics. Entropy is related to the number of microstates by \(S \propto \ln \Omega\). Since configuration B corresponds to a larger number of microstates and therefore higher entropy, the system is more likely to evolve from configuration A to configuration B than in the reverse direction.
