Mass defect and binding energy IB DP Physics Study Notes - 2025 Syllabus
Mass defect and binding energy IB DP Physics Study Notes
Mass defect and binding energy IB DP Physics Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on IB Physics syllabus with Students should understand
isotopes
nuclear binding energy and mass defect
the variation of the binding energy per nucleon with nucleon number
the mass-energy equivalence as given by E = mc2 in nuclear reactions
the existence of the strong nuclear force, a short-range, attractive force between nucleons
Standard level and higher level: 7 hours
Additional higher level: 5 hours
- IB DP Physics 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Physics 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Physics 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Physics 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Mass defect and nuclear binding energy
- To understand nuclear reactions, we use Einstein’s mass-energy equivalence relationship:

- It tells us that mass and energy are interchangeable.
Example:
If one kilogram of mass is completely converted into energy, how much energy is released?
▶️ Answer/Explanation
Using \( E = mc^2 \):
\( E = (1)(3.00 \times 10^8)^2 = 9.00 \times 10^{16} \, \text{J} \)
This equals approximately 21.5 megatons of TNT.
- In nuclear reactions, the total mass of the products is less than the total mass of the reactants. This difference is the mass defect \( \Delta m \).
- Using \( E = \Delta mc^2 \), we can find the nuclear binding energy: the energy holding the nucleus together.
Example:
Calculate the mass defect and binding energy of helium-4
▶️ Answer/Explanation
Constituent masses:
- 2 protons: \( 2 \times 1.007276 = 2.014552 \, \text{u} \)
- 2 neutrons: \( 2 \times 1.008665 = 2.017330 \, \text{u} \)
- Total expected mass: \( 4.031882 \, \text{u} \)
- Actual mass of He-4 nucleus: \( 4.002603 \, \text{u} \)
\( \Delta m = 4.031882 – 4.002603 = 0.029279 \, \text{u} \)
\( 1 \, \text{u} = 931.5 \, \text{MeV} \Rightarrow E_b = 0.029279 \times 931.5 = \boxed{27.3 \, \text{MeV}} \)
Example:
Binding energy of U-235 nucleus
▶️ Answer/Explanation
Protons: 92
Neutrons: 143
- Mass of 92 protons = \( 92 \times 1.007276 = 92.669392 \, \text{u} \)
- Mass of 143 neutrons = \( 143 \times 1.008665 = 144.238995 \, \text{u} \)
- Total expected mass: \( 236.908387 \, \text{u} \)
- Actual mass of U-235 nucleus = \( 235.043930 \, \text{u} \)
\( \Delta m = 236.908387 – 235.043930 = 1.864457 \, \text{u} \)
\( E_b = 1.864457 \times 931.5 = \boxed{1,735 \, \text{MeV}} \)
Binding energy per nucleon: \( \frac{1735}{235} \approx \boxed{7.39 \, \text{MeV}} \)
Example:
Binding energy of Carbon-12
▶️ Answer/Explanation
6 protons + 6 neutrons:
- Mass of protons = \( 6 \times 1.007276 = 6.043656 \, \text{u} \)
- Mass of neutrons = \( 6 \times 1.008665 = 6.051990 \, \text{u} \)
- Expected mass = \( 12.095646 \, \text{u} \)
- Actual mass = exactly 12.000000 u (by definition)
\( \Delta m = 12.095646 – 12.000000 = 0.095646 \, \text{u} \)
\( E_b = 0.095646 \times 931.5 = \boxed{89.1 \, \text{MeV}} \)
Per nucleon: \( \frac{89.1}{12} \approx \boxed{7.43 \, \text{MeV}} \)
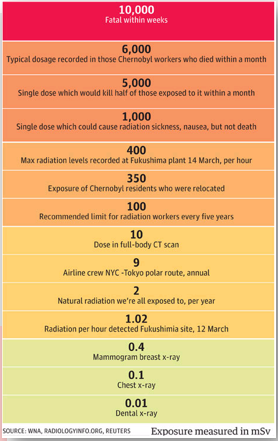
The ionizing effect of radiation

- High exposure: Damage to the central nervous system and death within weeks.
- Medium exposure: Damage to the stomach and intestines, leading to general sickness and diarrhea.
- Low exposure: Hair loss, bleeding, and diarrhea.
FYI
- There is evidence that cancer and genetic mutations can occur after exposure to radiation.
There are uses for radiation…
- X-rays of teeth and bones
- radiotherapy for cancer treatment
FYI
In radiotherapy radiation is used because rapidly dividing cancer cells are more susceptible to the damaging effects of radiation than healthy cells.
EXAMPLE:
What is the binding energy \(E_b\) in \(^4He\) in the nuclear reaction \(2(^1H) + 2n \rightarrow ^4He?\)
▶️Answer/Explanation
SOLUTION:
Since \(^4He\) is \(5.044 \times 10^{-29} \, \text{kg}\) lighter than \(2(^1H) + 2n,\) we need to provide enough energy to make the additional mass of the constituents.
\[
E_b = (5.044 \times 10^{-29})(3.00 \times 10^8)^2 = 4.54 \times 10^{-12} \, \text{J}.
\]
Thus, to pull apart the \(^4He\) and separate it into its constituents, \(4.54 \times 10^{-12} \, \text{J}\) are needed, which is the binding energy.