Nuclear Fusion IB DP Physics Study Notes - 2025 Syllabus
Nuclear FUssion IB DP Physics Study Notes
Nuclear Fusion IB DP Physics Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on IB Physics syllabus with Students should understand
that the stability of stars relies on an equilibrium between outward radiation pressure and inward gravitational forces
that fusion is a source of energy in stars
Standard level and higher level: 4 hours
Additional higher level: There is no additional higher level content.
- IB DP Physics 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Physics 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Physics 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Physics 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Mass defect and nuclear binding energy
A picture of the reaction 2(1H) + 2n → 4He might help:

∙The reverse process yields the same energy:

Solving problems involving mass defect and binding energy per nucleon
The binding energy per nucleon \( \frac{E_b}{A} \) of a nucleus is simply the binding energy \( E_b \) divided by the number of nucleons \( A \).
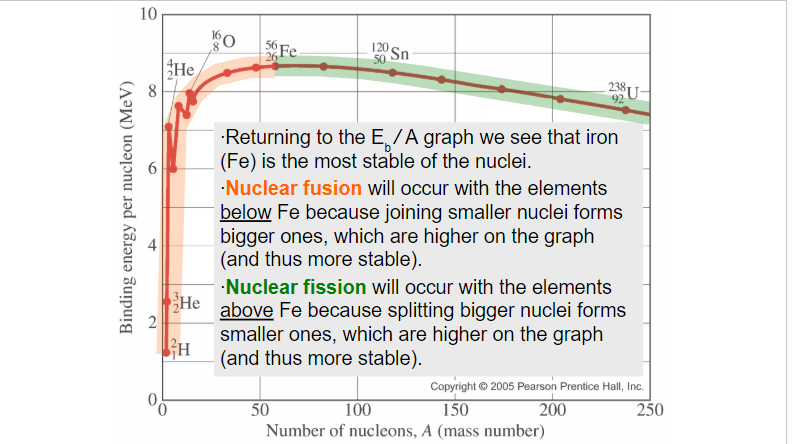
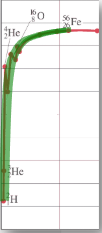
Sketching and interpreting the graph of average binding energy per nucleon against nucleon number
∙Rather than looking at the total binding energy of nuclei, we often look at the binding energy per nucleon.
∙This number tells us about how difficult it is to remove each nucleon from the nucleus.
∙The bigger the binding energy per nucleon, the more stable the nucleus.
FYI
∙The bigger the binding energy per nucleon, the less likely a nucleus will be to want to lose one of its nucleons.
∙Thus it is more stable, by definition.

Nuclear fission and nuclear fusion
∙Nuclear fission is the splitting of a large nucleus into two smaller (daughter) nuclei.
∙An example of fission is
![]()
∙In the animation, 235U is hit by a neutron, and capturing it, becomes excited and unstable:
∙It quickly splits into two smaller daughter nuclei, and two neutrons.

Note that the splitting was triggered by a single neutron that had just the right energy to excite the nucleus.
Example:
The mass of a helium-4 nucleus is \( 4.0015 \, \text{u} \). It contains 2 protons and 2 neutrons.
Given:
- Mass of 1 proton = \( 1.0073 \, \text{u} \)
- Mass of 1 neutron = \( 1.0087 \, \text{u} \)
▶️ Answer/Explanation
Step 1: Calculate total mass of separate nucleons
\( \text{Mass of 2 protons} = 2 \times 1.0073 = 2.0146 \, \text{u} \)
\( \text{Mass of 2 neutrons} = 2 \times 1.0087 = 2.0174 \, \text{u} \)
\( \text{Total mass of nucleons} = 2.0146 + 2.0174 = 4.0320 \, \text{u} \)
Step 2: Calculate mass defect
\( \Delta m = 4.0320 – 4.0015 = 0.0305 \, \text{u} \)
Step 3: Convert mass defect to energy
\( E = \Delta m \times 931.5 = 0.0305 \times 931.5 = \boxed{28.43 \, \text{MeV}} \)
Binding energy per nucleon:
\( \frac{28.43 \, \text{MeV}}{4} = \boxed{7.11 \, \text{MeV per nucleon}} \)
Nuclear fission and nuclear fusion
∙Nuclear fusion is the combining of two small nuclei into one larger nucleus.
∙An example of fusion is
∙In order to fuse two nuclei you must overcome the repulsive Coulomb force and get them close enough together that the strong force takes over.
∙Stars use their immense gravitational force to overcome the Coulomb force.
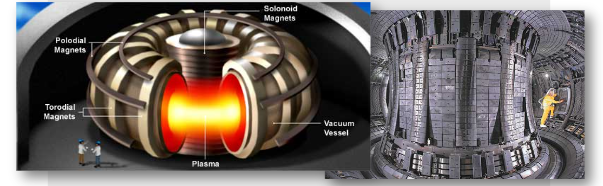
∙In our fusion reactors here on Earth, very precise and strong magnetic fields are used to overcome the Coulomb force.
∙So far their energy yield is less than their energy consumption and are thus still experimental.
∙The reason fusion works on stars is because their intense gravitational fields can overcome the Coulomb repulsion force between protons.
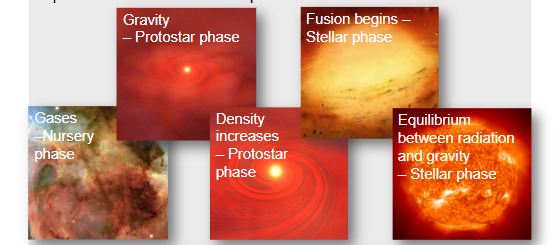
The gravitational force squishes hydrogen nuclei together overcoming the repulsive Coulomb force.
∙The energy released by fusion prevents the gravitational collapse from continuing.
∙Gravitation and radiation pressure reach equilibrium.
∙They balance each other.
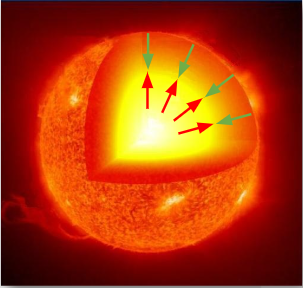
∙As the fusion progresses different elements evolve, and therefore different fusion reactions occur.
∙At the same time, the mass of the star decreases a bit according to E = mc2.
∙As a result, a new equilibrium is established between gravity and the radiation pressure and the star grows.
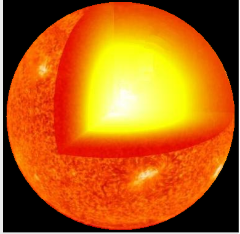
As the star evolves along the fusion side of the Eb / A curve, it slowly runs out of fuel to fuse and gravity again wins out.
∙The star begins to shrink.
∙For a star with the mass of the Sun, the shrinking will eventually stop and the sun will become a white dwarf and slowly cool down.
∙Gravity will not be strong enough to fuse any more of the nuclei.
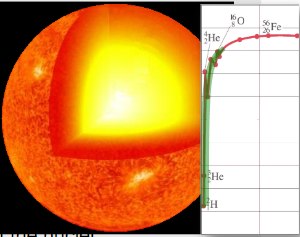
∙For a more massive star, there is enough gravity to fuse the elements all the way up to iron.
∙But there can be no more fusion when the star is completely iron. Why?
∙Since the radiation pressure now ceases, gravity is no longer balanced and the star collapses into a neutron star.
∙This is called the iron catastrophe.
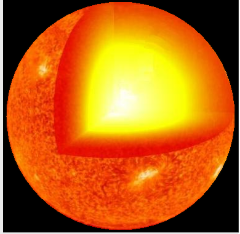
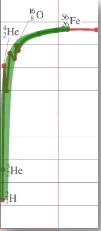
∙For an even more massive star, there is enough gravity to overcome the neutron barrier to collapse.
∙Collapse continues and nothing can stop it!
∙The star becomes a black hole.
∙A black hole is an extremely dense body whose gravitational force is so strong that even light cannot escape it!

∙Thus you cannot see it directly.
Example:
In a nuclear reactor, Uranium-235 undergoes fission when struck by a neutron:
\( \ce{^{235}_{92}U + ^{1}_{0}n -> ^{144}_{56}Ba + ^{89}_{36}Kr + 3 ^{1}_{0}n} \)
▶️ Answer/Explanation
∙ The Uranium-235 nucleus absorbs a neutron and becomes unstable.
∙ It splits into two smaller nuclei: Barium-144 and Krypton-89, and releases 3 more neutrons.
∙ This process releases energy due to the mass defect—converted into kinetic energy and radiation.
∙ The emitted neutrons can go on to trigger further fission reactions, causing a chain reaction.
Final Result:
\( \boxed{\text{Large energy release and neutron chain reaction}} \)
Example:
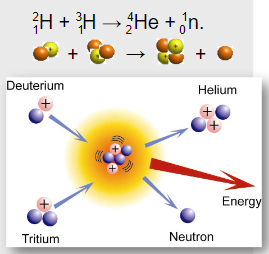
In the Sun, hydrogen nuclei fuse to form helium:
\( \ce{^{2}_{1}H + ^{3}_{1}H -> ^{4}_{2}He + ^{1}_{0}n} \)
▶️ Answer/Explanation
∙ A deuterium nucleus (\( \ce{^{2}_{1}H} \)) fuses with a tritium nucleus (\( \ce{^{3}_{1}H} \)).
∙ This forms a helium-4 nucleus and a free neutron.
∙ The total mass of the products is slightly less than the mass of the reactants—this mass defect is released as energy.
Final Result:
\( \boxed{\text{Mass converted to energy; powers stars like the Sun}} \)
IB Physics Nuclear Fusion Exam Style Worked Out Questions
Question
A gamma ray can split into an electron and a positron when it passes through certain materials. Which process describes this phenomenon?
A. Pair production
B. Pair annihilation
C. Nuclear fission
D. Radioactive decay
▶️Answer/Explanation
Ans:A

The phenomenon in which a gamma ray can split into an electron and a positron when passing through certain materials is described as “Pair production.” Pair production is a process in which a gamma ray of sufficient energy is converted into an electron and a positron. A fundamental law of mechanics, given by Newton, is that in any process total linear (as well as angular) momentum remains unchanged.
