Photons and the photoelectric effect IB DP Physics Study Notes - 2025 Syllabus
Photons and the photoelectric effect IB DP Physics Study Notes
Photons and the photoelectric effect IB DP Physics Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on IB Physics syllabus with Students should understand
the photoelectric effect as evidence of the particle nature of light
that photons of a certain frequency, known as the threshold frequency, are required to release photoelectrons from the metal
Einstein’s explanation using the work function and the maximum kinetic energy of the photoelectrons as given by Emax = hƒ–Φ where Φ is the work function of the metal
diffraction of particles as evidence of the wave nature of matter
Standard level and higher level: There is no standard level content
Additional higher level: 8 Hours
- IB DP Physics 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Physics 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Physics 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Physics 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
The quantum nature of radiation
∙Back in the very early 1900s physicists thought that within a few years everything having to do with physics would be discovered and the “book of physics” would be complete.
∙This “book of physics” has come to be known as classical physics and consists of particles and mechanics on the one hand, and wave theory on the other.
∙Two men who spearheaded the physics revolution which we now call modern physics were Max Planck and Albert Einstein.
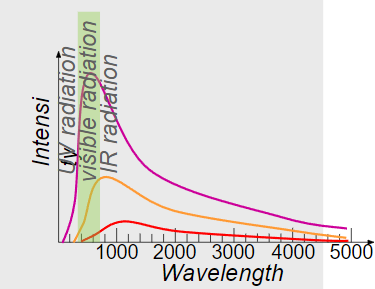
∙To understand Planck’s contribution to modern physics we revisit black- body radiation and its characteristic curves:
∙Recall Wien’s displacement law which gives the relationship between the wavelength and intensity for different temperatures.
![]()
FYI
∙Note that the intensity becomes zero for very long and very short wavelengths of light.
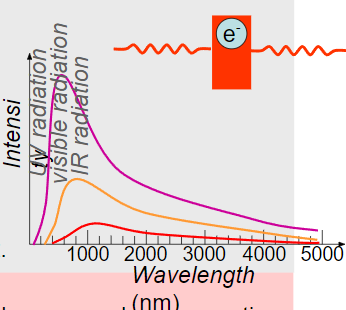
∙Blackbody radiation gave Planck the first inkling that things were not as they should be.
∙As far as classical wave theory goes, thermal radiation is caused by electric charge acceleration near the surface of an object.
FYI
∙Recall that moving electric charges produce magnetic fields. Accelerated electric charges produce electromagnetic radiation, including visible light.
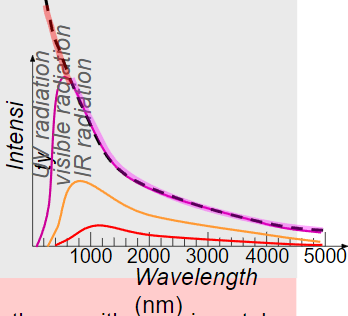
∙According to classical wave theory, the intensity vs. wavelength curve should look like the dashed line:
∙For long wavelengths the predicted and observed curves match up well.
∙But for small wavelengths, classical theory fails.
FYI
∙The failure of classical wave theory with experimental observation of blackbody radiation was called the ultraviolet catastrophe.

∙In 1900, the UV catastrophe led German physicist Max Planck to reexamine blackbody radiation.
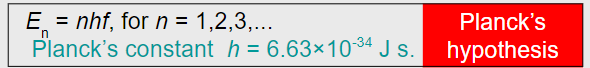
∙Planck discovered that the failure of classical theory was in assuming that energy could take on any value (in other words, that it was continuous).
∙Planck hypothesized that if thermal oscillators could only vibrate at specific frequencies delivering packets of energy he called quanta, then the ultraviolet catastrophe was resolved.
EXAMPLE:
Using Planck’s hypothesis, show that the energy \( E \) of a single quantum with frequency \( f \) is given by
$E = \frac{hc}{\lambda}. $
Find the energy contained in a single quantum of light having a wavelength of \( 500 \, \text{nm}. \)
▶️Answer/Explanation
SOLUTION:
From classical wave theory, \( v = \lambda f \).
But for light, \( v = c \), the speed of light.
Thus \( f = \frac{c}{\lambda} \) and we have \( E = hf = \frac{hc}{\lambda} \).
\[
E = \frac{hc}{\lambda}
\]
For light having a wavelength of \( 500 \, \text{nm} \), we have:
\[
E = \frac{hc}{\lambda} = \frac{(6.63 \times 10^{-34})(3.00 \times 10^8)}{500 \times 10^{-9}} = 3.98 \times 10^{-19} \, \text{J}.
\]
The photoelectric effect

∙In the early 1900s Albert Einstein conducted experiments in which he irradiated photosensitive metals with light of different frequencies and intensities.
∙In 1905 he published a paper on the photoelectric effect, in which he postulated that energy quantization is also a fundamental property of electromagnetic waves (including visible light and heat).
∙He called the energy packet a photon, and postulated that light acted like a particle as well as a wave.
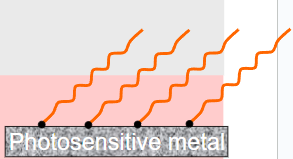
Certain metals are photosensitive – meaning that when they are struck by radiant energy, they emit electrons from their surface.
∙In order for this to happen, the light must have done work on the electrons.
FYI
∙Perhaps the best-known example of an application using photosensitive metals is photocopy machine.
∙Light reflects off of a document causing a charge on the photosensitive drum in proportion to the color and intensity of the light reflected.
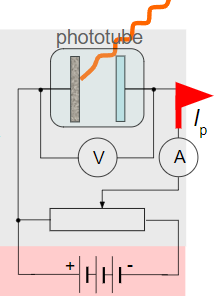
∙Einstein enhanced the photoelectric effect by placing a plate opposite and applying a potential difference:
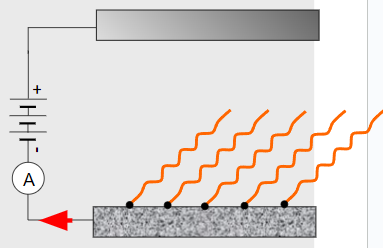
∙The positive plate attracts the photo- electrons whereas the negative plate repels them.
∙From the reading on the ammeter he could determine the current of the photoelectrons.
∙If he reversed the polarity of the plates, Einstein found that he could adjust the voltage until the photocurrent stopped.
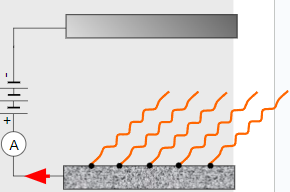
∙The top plate now repels the photoelectrons whereas the bottom plate attracts them back.
∙The ammeter now reads zero because there is no longer a photocurrent.
∙The experimental setup is shown:
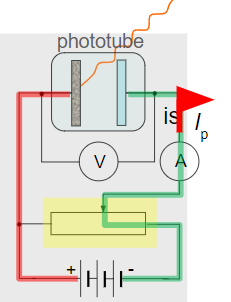
∙Monochromatic light of fixed intensity is shone into the tube, creating a photocurrent Ip.
∙Note the reversed polarity of the plates and the potential divider that is used to adjust the voltage.
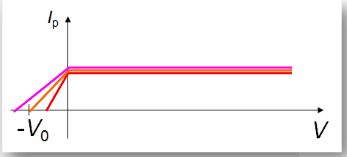
∙Ip remains constant for all positive p.d.’s.
∙Not until we reach a p.d. of zero, and start reversing the polarity, do we see a response:

We call the voltage –V0 at which Ip becomes zero the cutoff voltage.
∙Einstein discovered that if the intensity were increased, even though Ip increased substantially, the cutoff voltage remained V0.
FYI
∙Classical theory predicts that increased intensity should produce higher Ip.
∙But classical theory also predicts that the cutoff voltage should change when it obviously doesn’t.
Einstein also discovered that if the frequency of the light delivering the photons increased, so did the cutoff voltage.
∙Einstein noted that if the frequency of the light was low enough, no matter how intense the light no photocurrent was observed. He termed this minimum frequency needed to produce a photocurrent the cutoff frequency f0.
∙And finally, he observed that if the frequency was above f0, even if the intensity was extremely low, the photocurrent would begin immediately.
∙Einstein found that if he treated light as if it were a stream of particles instead of a wave that his theory could predict all of the observed results of the photoelectric effect.
∙The light particle (photon) has the same energy as Planck’s quantum of thermal oscillation:

∙Einstein defined a work function φ which was the minimum amount of energy needed to “knock” an electron from the metal. A photon having a frequency at least as great as the cutoff frequency f0 was needed.
![]()
∙If an electron was freed by the incoming photon having energy E = hf, and if it had more energy than the work function, the electron would have a maximum kinetic energy in the amount of
![]()
∙Putting it all together into a single formula:

Example:
When ultraviolet light of wavelength \( \lambda = 250 \, \text{nm} \) is incident on a clean zinc surface, photoelectrons are emitted. The work function of zinc is \( \phi = 4.3 \, \text{eV} \). Calculate the maximum kinetic energy of the emitted electrons and the stopping potential required to stop them.
▶️ Answer/Explanation
Use Planck’s equation to find the energy of the incident photons
\( E = \frac{hc}{\lambda} \)
Where:
- \( h = 6.63 \times 10^{-34} \, \text{J·s} \)
- \( c = 3.00 \times 10^8 \, \text{m/s} \)
- \( \lambda = 250 \, \text{nm} = 250 \times 10^{-9} \, \text{m} \)
\( E = \frac{(6.63 \times 10^{-34})(3.00 \times 10^8)}{250 \times 10^{-9}} \)
\( E = 7.95 \times 10^{-19} \, \text{J} \)
Convert to electronvolts: \( 1 \, \text{eV} = 1.6 \times 10^{-19} \, \text{J} \)
\( E = \frac{7.95 \times 10^{-19}}{1.6 \times 10^{-19}} = 4.97 \, \text{eV} \)
Use Einstein’s photoelectric equation
\( K_{\text{max}} = E_{\text{photon}} – \phi \)
\( K_{\text{max}} = 4.97 \, \text{eV} – 4.3 \, \text{eV} = 0.67 \, \text{eV} \)
Find the stopping potential \( V_s \)
Since \( K_{\text{max}} = eV_s \), and \( e = 1 \, \text{eV} \) per volt,
\( V_s = 0.67 \, \text{V} \)
IB Physics Photons and the photoelectric effect Exam Style Worked Out Questions
Question
In a photoelectric experiment a stopping voltage $V$ required to prevent photoelectrons from flowing across the photoelectric cell is measured for light of two frequencies $f_1$ and $f_2$. The results obtained are shown.
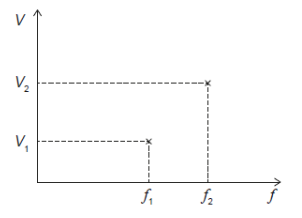
The ratio $\frac{V_2-V_1}{f_2-f_1}$ is an estimate of
A. $e$
B. $h$
C. $\frac{e}{h}$
D. $\frac{h}{e}$
▶️Answer/Explanation
Ans:D
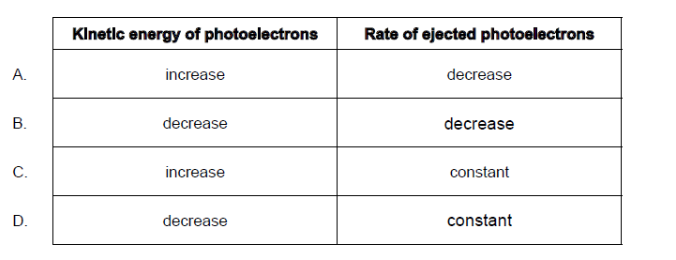
Question
A metallic surface is first irradiated with infrared radiation and photoelectrons are emitted from the surface. The infrared radiation is replaced by ultraviolet radiation of the same intensity.
What will be the change in the kinetic energy of the photoelectrons and the rate at which they are ejected?
▶️Answer/Explanation
Ans:A
