IBDP Chemistry - Reactivity 1.3 Energy from fuels- IB Style Questions For SL Paper 1A - FA 2025
Question
\(C_{2}H_{6}(g)+Cl_{2}(g)\rightarrow C_{2}H_{5}Cl(g)+HCl(g)\)
\((C_{2}H_{6}=30.08, Cl_{2}=70.90, C_{2}H_{5}Cl=64.52, HCl=36.46 \text{ g mol}^{-1})\)
What is the atom economy for this reaction?
(B) \(46.6\%\)
(C) \(63.9\%\)
(D) \(99.9\%\)
▶️ Answer/Explanation
1. Atom Economy Formula:
\(\text{Atom Economy} = \frac{\text{Molar Mass of Desired Product}}{\text{Total Molar Mass of All Reactants}} \times 100\%\)
2. Identify Masses:
- Desired Product: Chloroethane (\(C_2H_5Cl\)). Mass = \(64.52\) g/mol.
- Total Reactants: \(C_2H_6 + Cl_2\). Mass = \(30.08 + 70.90 = 100.98\) g/mol. (Alternatively, mass of all products = \(64.52 + 36.46 = 100.98\) g/mol).
3. Calculate:
\(\text{Atom Economy} = \frac{64.52}{100.98} \times 100\%\)
\(\approx 0.6389 \times 100\% = 63.9\%\)
✅ Answer: (C)
Question
\(CH_{3}CH_{2}CH_{3}(g)+5O_{2}(g)\rightarrow3CO_{2}(g)+4H_{2}O(l)\)
What is the volume of the remaining unreacted oxygen under the initial conditions?
(B) \(50\) cm\(^3\)
(C) \(75\) cm\(^3\)
(D) \(150\) cm\(^3\)
▶️ Answer/Explanation
1. Use Avogadro’s Law:
At constant temperature and pressure, volume is proportional to moles. We can use volumes directly in stoichiometric calculations.
2. Calculate Required Oxygen:
Reaction ratio: \(1\) vol Propane reacts with \(5\) vols Oxygen.
Required \(O_2\) = \(5 \times 75 \text{ cm}^3 = 375 \text{ cm}^3\).
3. Determine Remaining Oxygen:
Available \(O_2\) = \(400 \text{ cm}^3\).
Remaining \(O_2\) = Available – Consumed
Remaining \(O_2\) = \(400 – 375 = 25 \text{ cm}^3\).
✅ Answer: (A)
Question
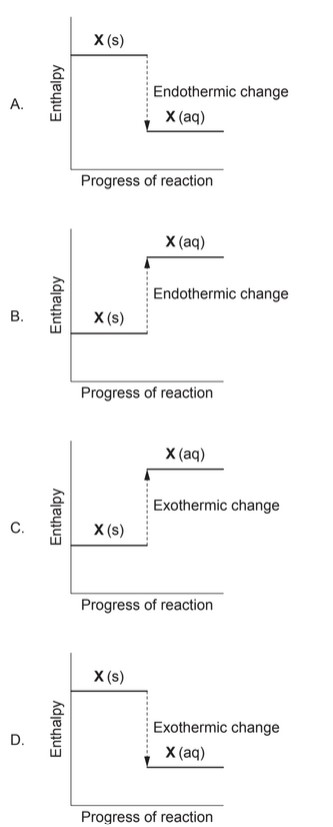
▶️ Answer/Explanation
When the temperature of the solution decreases, the process must be endothermic: the dissolving absorbs heat from the surroundings (the solution), causing its temperature to drop.
In an endothermic process the enthalpy of the products is greater than that of the reactants: \[ \Delta H > 0 \quad \Rightarrow \quad H_{\text{products}} > H_{\text{reactants}}. \] So \(X(aq)\) must lie at a higher enthalpy level than \(X(s)\).
Only diagram B shows \(X(s)\) at a lower enthalpy and \(X(aq)\) at a higher enthalpy with the change labelled “Endothermic change”.
✅ Answer: (B)
