IBDP Chemistry - Reactivity 2.2 How fast? The rate of chemical change- IB Style Questions For SL Paper 1A - FA 2025
Question
▶️ Answer/Explanation
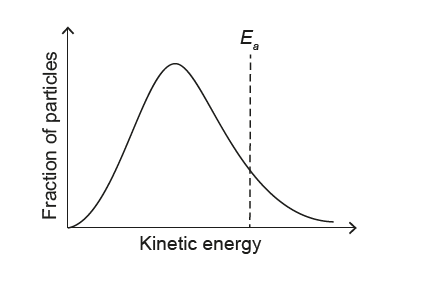
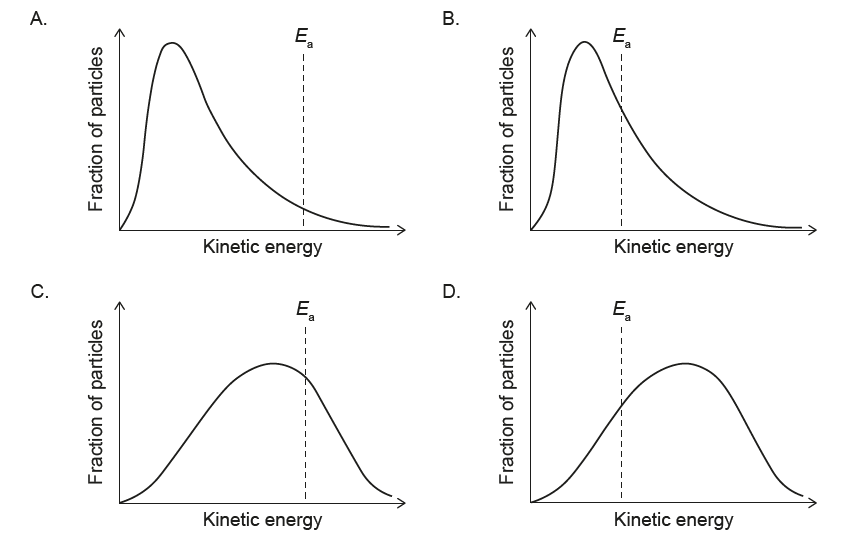
1. Effect of lowering temperature on the Maxwell–Boltzmann distribution:
When the temperature decreases, the average kinetic energy of the particles decreases.
The Maxwell–Boltzmann curve becomes:
– shifted to the left (towards lower kinetic energy), and
– higher and narrower (more particles with energies close to the new, lower mean).
2. Effect on the activation energy \(E_{a}\):
The activation energy \(E_{a}\) is a fixed property of the reaction and does not change with temperature. Therefore, the vertical line representing \(E_{a}\) must stay at the same kinetic energy value on the horizontal axis.
The only option that shows a narrower, taller curve shifted to lower kinetic energy while keeping the \(E_{a}\) line in the same position is diagram A.
✅ Answer: (A)
Question
The following graph shows the concentration of \( C_{4}H_{9}Cl \) versus time.

B. \( 1 \times 10^{-4} \text{ mol dm}^{-3}\text{ s}^{-1} \)
C. \( 2 \times 10^{-3} \text{ mol dm}^{-3}\text{ s}^{-1} \)
D. \( 2 \times 10^{-4} \text{ mol dm}^{-3}\text{ s}^{-1} \)
▶️ Answer/Explanation
The rate is calculated using:
\[ \text{rate} = -\frac{[\text{reactant at } t_2] – [\text{reactant at } t_1]}{t_2 – t_1} \] Over the first \( 800 \) seconds:
\[ \text{rate} = -\frac{0.10 – 0.02}{0 – 800} \Rightarrow 1 \times 10^{-4} \]
✅ Correct answer: B
Question
Consider the reaction between gaseous iodine and gaseous hydrogen.
Why do some collisions between iodine and hydrogen not result in the formation of the product?
B. The system is in equilibrium.
C. The temperature of the system is too high.
D. The activation energy for this reaction is very low.
▶️ Answer/Explanation
For a successful collision, reacting particles must collide with sufficient energy to overcome the activation energy barrier. Many \( \mathrm{I_2} \) and \( \mathrm{H_2} \) molecules collide with less than this minimum energy, so they simply bounce off each other without forming \( \mathrm{HI} \).
✅ Correct answer: A
