IBDP Chemistry -Reactivity 3.1 Proton transfer reactions - IB Style Questions For SL Paper 2 -FA 2025
Question
\( \text{Co}^{2+}(aq) + 4\text{Cl}^-(aq) \rightleftharpoons \text{CoCl}_4^{2-}(aq) \)
(Pink) \(\rightleftharpoons\) (Blue)
(i) Write the expression for the equilibrium constant, \(K\), for this reaction.
(ii) Predict the effect on both the value of \(K\) and the position of equilibrium when solid sodium chloride, \(\text{NaCl}(s)\), is added at constant temperature.
(iii) On heating, a mixture that is initially pink becomes purplish-blue. Deduce, with a reason, whether the formation of \(\text{CoCl}_4^{2-}(aq)\) is exothermic or endothermic.
(iv) Draw an energy profile diagram for this equilibrium, clearly labelling the reactants, products, activation energy, \(E_a\), and the enthalpy change for the forward reaction, \(\Delta H\).
Most-appropriate topic codes (Chemistry):
• TOPIC R3.1: The equilibrium law — part (b-i)
• TOPIC R3.2: Le Châtelier’s principle — parts (b-ii), (b-iii)
• TOPIC R1.2: Energy cycles in reactions — part (b-iv)
▶️ Answer/Explanation
(a)
\(1s^2 2s^2 2p^6 3s^2 3p^6 3d^7\) OR \([\text{Ar}] 3d^7\). (Electrons are removed from \(4s\) first).
(b)
(i) \[ K = \frac{[\text{CoCl}_4^{2-}]}{[\text{Co}^{2+}][\text{Cl}^-]^4} \]
(ii)
• Value of K: No effect (K changes only with temperature).
• Equilibrium position: Shifts to the right (towards products) because adding \(\text{NaCl}\) increases \([\text{Cl}^-]\), driving the reaction forward to consume it.
(iii) Endothermic. Heating increases the temperature, which turns the mixture blue (product side). According to Le Châtelier’s principle, the system shifts to absorb heat, so the forward reaction must be endothermic.
(iv) The energy profile should show:
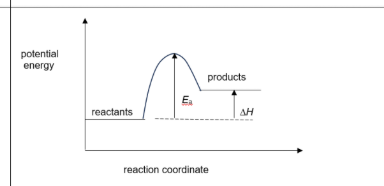
• Reactants at a lower energy level than products.
• \(E_a\) arrow from the reactant line to the peak of the curve.
• \(\Delta H\) arrow pointing upwards from reactant line to product line.
