IBDP Chemistry -Reactivity 3.2 Electron transfer reactions - IB Style Questions For SL Paper 2 -FA 2025
Question
Most-appropriate topic codes (IB Chemistry 2025):
• Reactivity 3.2: Le Châtelier’s principle and changes in conditions — part (b)
▶️ Answer/Explanation
(a)
1. Equilibrium concentrations:
\( [\text{N}_2] = \frac{30.0}{55.0} = 0.545\ \text{mol dm}^{-3} \)
\( [\text{H}_2] = \frac{40.0}{55.0} = 0.727\ \text{mol dm}^{-3} \)
\( [\text{NH}_3] = \frac{6.17}{55.0} = 0.112\ \text{mol dm}^{-3} \)
2. Equilibrium expression:
\( K = \frac{[\text{NH}_3]^2}{[\text{N}_2][\text{H}_2]^3} \)
3. Substitution and calculation:
\( K = \frac{(0.112)^2}{(0.545)(0.727)^3} = 0.0599 \)
\(\boxed{0.0599}\)
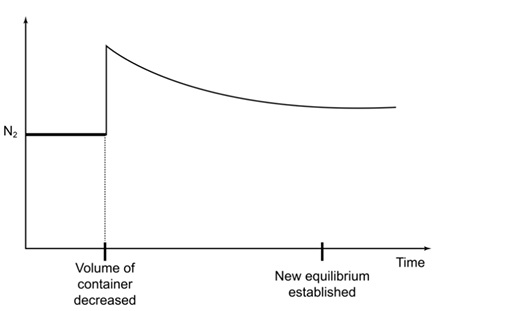
• When the volume is decreased, the concentration of \( \text{N}_2 \) increases suddenly due to compression.
• The system then shifts to the right, towards the side with fewer gas molecules, causing \( [\text{N}_2] \) to decrease gradually.
• A new equilibrium is reached where the nitrogen concentration is higher than the original equilibrium value but lower than the immediate post-compression value.
Sharp increase → gradual decrease → new constant level above the initial concentration.