IBDP Chemistry - Reactivity 3.3 Electron sharing reactions- IB Style Questions For SL Paper 1A - FA 2025
Question
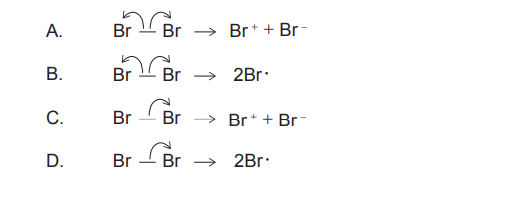
▶️ Answer/Explanation
1. Define Heterolytic Fission:
The bond breaks unevenly. One atom takes both electrons from the shared pair, forming ions (cation + anion). This is represented by a double-headed arrow (curly arrow) originating from the bond and pointing to the atom gaining the electrons.
2. Define Homolytic Fission:
The bond breaks evenly. Each atom gets one electron, forming radicals. Represented by single-headed (fish-hook) arrows.
3. Evaluate Options (Visual check):
- Option C shows a double-headed curly arrow moving the electron pair to one Br, forming \(Br^+\) and \(Br^-\). This represents heterolytic fission.
- Options B and D show fish-hook arrows (homolytic).
✅ Answer: (C)
Question
B. \(\cdot\text{C}_{2}\text{H}_{5} + \text{Cl}_{2} \rightarrow \text{C}_{2}\text{H}_{5}\text{Cl} + \cdot\text{Cl}\)
C. \(\cdot\text{C}_{2}\text{H}_{5} + \cdot\text{Cl} \rightarrow \text{C}_{2}\text{H}_{5}\text{Cl}\)
D. \(\text{C}_{2}\text{H}_{6} + \cdot\text{Cl} \rightarrow \text{C}_{2}\text{H}_{5}\text{Cl} + \cdot\text{H}\)
▶️ Answer/Explanation
In a free-radical substitution mechanism:
• Initiation produces radicals from non-radicals.
• Propagation steps use a radical and produce a new radical.
• Termination steps remove radicals by combining them.
Option A: \(\text{Cl}_{2} \rightarrow 2\,\cdot\text{Cl}\) produces radicals from a molecule → this is an initiation step.
Option B: \(\cdot\text{C}_{2}\text{H}_{5} + \text{Cl}_{2} \rightarrow \text{C}_{2}\text{H}_{5}\text{Cl} + \cdot\text{Cl}\)
A radical reacts and another radical is formed → this is a propagation step.
Option C: \(\cdot\text{C}_{2}\text{H}_{5} + \cdot\text{Cl} \rightarrow \text{C}_{2}\text{H}_{5}\text{Cl}\) removes two radicals → this is a termination step.
Option D: \(\text{C}_{2}\text{H}_{6} + \cdot\text{Cl} \rightarrow \text{C}_{2}\text{H}_{5}\text{Cl} + \cdot\text{H}\) is a possible propagation step in some mechanisms, but for the standard ethane–chlorine mechanism, the key accepted propagation steps are:
\(\text{C}_{2}\text{H}_{6} + \cdot\text{Cl} \rightarrow \cdot\text{C}_{2}\text{H}_{5} + \text{HCl}\)
\(\cdot\text{C}_{2}\text{H}_{5} + \text{Cl}_{2} \rightarrow \text{C}_{2}\text{H}_{5}\text{Cl} + \cdot\text{Cl}\).
Only option B matches one of these exactly.
✅ Answer: (B)
Question
II. \(\cdot\text{C}_{2}\text{H}_{5} + \text{Br}_{2} \rightarrow \text{C}_{2}\text{H}_{5}\text{Br} + \cdot\text{Br}\)
III. \(\cdot\text{C}_{2}\text{H}_{5} + \cdot\text{Br} \rightarrow \text{C}_{2}\text{H}_{5}\text{Br}\)
B. I and III only
C. II and III only
D. I, II and III
▶️ Answer/Explanation
In a free-radical halogenation mechanism:
• Initiation: radicals are produced from a non-radical (e.g. \(\text{Br}_{2} \xrightarrow{h\nu} 2\,\cdot\text{Br}\)).
• Propagation: a radical reacts to form a new radical, keeping the chain going.
• Termination: two radicals combine to form a stable molecule, removing radicals.
Step I: \(\text{C}_{2}\text{H}_{6} + \cdot\text{Br} \rightarrow \cdot\text{C}_{2}\text{H}_{5} + \text{HBr}\) – radical + molecule → new radical + molecule ⇒ propagation.
Step II: \(\cdot\text{C}_{2}\text{H}_{5} + \text{Br}_{2} \rightarrow \text{C}_{2}\text{H}_{5}\text{Br} + \cdot\text{Br}\) – radical + molecule → new radical + molecule ⇒ another propagation step.
Step III: \(\cdot\text{C}_{2}\text{H}_{5} + \cdot\text{Br} \rightarrow \text{C}_{2}\text{H}_{5}\text{Br}\) – two radicals combine to form a stable product ⇒ a termination step.
The full mechanism of bromination includes both propagation (I and II) and termination (III), so all three steps are involved.
✅ Answer: (D)
