IBDP Chemistry -Structure 1.3 Electron configurations- IB Style Questions For SL Paper 1A -FA 2025
Question
▶️ Answer/Explanation
1. Find the total number of electrons for each species:
\(W^{2+}:\) atomic number \(12 \Rightarrow\) electrons \(= 12 – 2 = 10\).
\(X^{-}:\) atomic number \(16 \Rightarrow\) electrons \(= 16 + 1 = 17\).
\(Y^{2+}:\) atomic number \(21 \Rightarrow\) electrons \(= 21 – 2 = 19\).
\(Z^{-}:\) atomic number \(9 \Rightarrow\) electrons \(= 9 + 1 = 10\).
2. Compare electron arrangements and outer electrons:
Species with \(10\) electrons have the same configuration as neon: \(2, 8\), so they each have \(8\) outer (valence) electrons.
Thus \(W^{2+}\) and \(Z^{-}\) both possess \(8\) valence electrons.
The species with \(17\) and \(19\) electrons have different outer-electron counts.
✅ Answer: (D)
Question
▶️ Answer/Explanation
The maximum number of electrons that can occupy a principal energy level \(n\) is given by: \[ \text{max electrons} = 2n^{2} \]
For \(n = 4\): \[ \text{max electrons} = 2 \times 4^{2} = 2 \times 16 = 32 \]
Therefore, the maximum number of electrons in energy level \(n = 4\) is \(\,32\), which corresponds to option C.
✅ Answer: (C)
Question
What is the correct ground state electron orbital configuration for \(2s^2 2p^2\)?
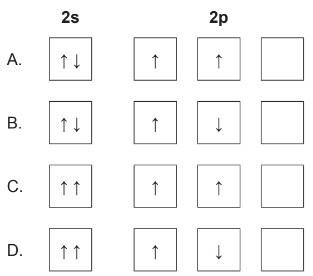
▶️ Answer/Explanation
The configuration \(2s^2 2p^2\) corresponds to carbon. The \(2s\) orbital contains a paired set of electrons: \[ 2s: \uparrow\downarrow \]
The \(2p\) orbitals each receive one electron before any pairing occurs, following Hund’s rule: \[ 2p: \uparrow\ \ \uparrow\ \ \Box \]
This corresponds exactly to option A.
✅ Answer: (A)
