IBDP Chemistry -Structure 2.3 The metallic model - IB Style Questions For SL Paper 2 -FA 2025
Question
(a) A fragment of an addition polymer is shown below.
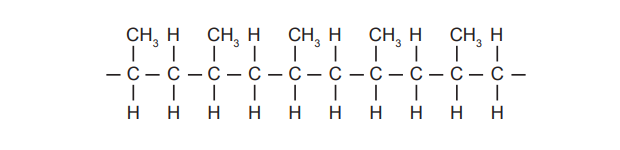
(i) Deduce the structure of the monomer from which this polymer is formed.
(ii) Describe one chemical property that makes this type of polymer suitable for practical use.
(b) The organic compound shown, labelled \(X\) (3,5,5-trimethylhexanal), is used as a flavouring substance.
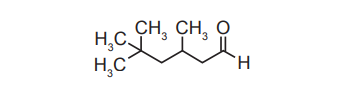
(i) Identify the functional group present in \(X\).
(ii) Deduce the systematic IUPAC name of \(X\).
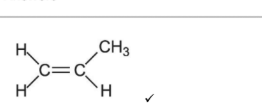
(iii) Draw an isomer of \(X\) that belongs to a different homologous series.
(iv) Molecule \(X\) can undergo both oxidation and reduction. Using RCHO to represent \(X\), deduce the formulas of the organic products formed when \(X\) reacts separately with an oxidizing agent and with a reducing agent.
(c) An alkene such as ethene can act as a starting material for the synthesis of many compounds.
(i) Predict the product formed when ethene reacts with bromine.
(ii) Outline why unsaturated molecules, such as ethene, readily undergo this type of reaction.
(iii) State the general formula for the homologous series of alkenes.
(iv) Explain, with reference to intermolecular forces, the trend in the boiling points of the first four alkenes.
| Alkene | Boiling point / K |
|---|---|
| ethene | \(169\) |
| propene | \(225\) |
| but-1-ene | \(267\) |
| pent-1-ene | \(303\) |
\[ \text{C}_2\text{H}_4(g) + \text{H}_2\text{O}(g) \rightarrow \text{C}_2\text{H}_5\text{OH}(g) \]
Calculate the enthalpy change of this reaction, in kJ, using section 12 of the data booklet.
Most-appropriate topic codes (Chemistry):
• TOPIC R2.1: Electron transfer reactions — part (b-iv)
• TOPIC S2.3: Intermolecular forces — part (c-iv)
• TOPIC R1.2: Energy cycles — part (d)
▶️ Answer/Explanation
(a)
(i) The monomer is propene. Structure: \(\text{H}_2\text{C}=\text{CH}-\text{CH}_3\).
(ii) It is chemically inert or unreactive (or non-biodegradable), making it durable.
(b)
(i) Carbonyl.
(ii) 3,5,5-trimethylhexanal.
(iii) Any isomer with formula \(\text{C}_9\text{H}_{18}\text{O}\) that is a ketone, cyclic alcohol, or unsaturated alcohol. 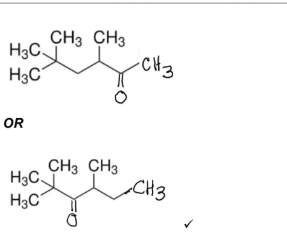
(iv)
• Oxidation: \(\text{RCOOH}\) (carboxylic acid)
• Reduction: \(\text{RCH}_2\text{OH}\) (primary alcohol)
(c)
(i) 1,2-dibromoethane.
(ii) The high electron density of the carbon–carbon double bond makes it susceptible to electrophilic attack (or because the C=C bond allows addition reactions).
(iii) \(\text{C}_n\text{H}_{2n}\).
(iv) The boiling points increase from \(169\ \text{K}\) to \(303\ \text{K}\) as chain length (and molar mass) increases, leading to stronger London (dispersion) forces due to a larger electron cloud and surface area.
(d)
Enthalpy change \(\Delta H = \Sigma(\text{bonds broken}) – \Sigma(\text{bonds formed})\).
• Bonds broken: \(1 \times (\text{C}=\text{C}) + 1 \times (\text{O}-\text{H}) = 614 + 463 = 1077\ \text{kJ}\).
• Bonds formed: \(1 \times (\text{C}-\text{C}) + 1 \times (\text{C}-\text{O}) + 1 \times (\text{C}-\text{H}) = 346 + 358 + 414 = 1118\ \text{kJ}\).
\[ \Delta H = 1077 – 1118 = \mathbf{-41\ \text{kJ}} \] (Alternatively, total bonds: broken \(= 3196\), formed \(= 3237\); \(3196 – 3237 = -41\)).
