IBDP Chemistry - Structure 3.2 Functional groups: Classification of organic compounds- IB Style Questions For SL Paper 1A - FA 2025
▶️ Answer/Explanation
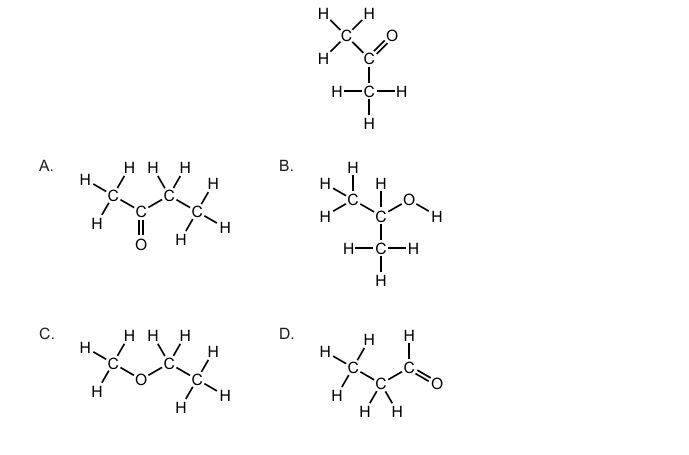
Propanone has the molecular formula C₃H₆O. Isomers must have the same molecular formula but different structural arrangements. Among the given options, the compound that has the formula C₃H₆O with a different functional group or carbon skeleton is the correct isomer.
✅ Answer: (D)
Question
B. Determination of melting point
C. Infra-red (IR) spectroscopy
D. Mass spectroscopy (MS)
▶️ Answer/Explanation
Functional groups are specific bonding patterns such as O–H, C=O, N–H, etc. Each of these groups absorbs infra-red radiation at characteristic frequencies because their bonds vibrate (stretch and bend) at particular energies.
• Infra-red (IR) spectroscopy measures which wavelengths are absorbed, producing an IR spectrum with peaks that can be matched to known functional-group absorptions (for example, a strong broad peak around \( 3200\text{–}3600 \,\text{cm}^{-1} \) for O–H, or a sharp peak near \( 1700 \,\text{cm}^{-1} \) for C=O). This makes IR especially useful for identifying which functional groups are present in a molecule.
• Combustion analysis gives empirical formula (percentage of C, H, sometimes O), but not which functional groups are present.
• Determination of melting point is used mainly for checking purity or identifying a compound by comparison to known data, not for specific functional groups.
• Mass spectroscopy (MS) is excellent for finding molar mass and fragment patterns, but it does not directly pinpoint functional-group absorptions in the way IR does.
Therefore, the technique most likely to be used for identification of functional groups is Infra-red (IR) spectroscopy.
✅ Answer: C
Question

B. 2,3-dimethylbutan-2-ol
C. 1-ethyl-2-methylpropan-1-ol
D. 1,1,2-trimethylpropan-1-ol
▶️ Answer/Explanation
1. First choose the longest carbon chain that includes the carbon bearing the \(-\text{OH}\) group. In the given structure this chain has four carbons, so the parent is butan-2-ol (the \(-\text{OH}\) is on carbon 2).
2. Now identify substituents on this four-carbon chain. – There is a methyl substituent on carbon 2. – There is another methyl substituent on carbon 3.
3. Combining this information, the correct IUPAC name is 2,3-dimethylbutan-2-ol, which corresponds to option B.
4. The other options are incorrect because they either use a shorter parent chain or place the substituents and \(-\text{OH}\) group at the wrong positions.

✅ Answer: B