Energy cycles in reactions: R1.2.1 Bond enthalpy IB DP Chemistry Study Notes - New Syllabus 2025
Energy cycles in reactions – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 1.2.1 – Bond Enthalpy
Reactivity 1.2.1 – Bond Enthalpy
Energy Changes in Chemical Reactions
Chemical reactions involve changes in the bonding between atoms. During a chemical reaction:
- Bonds in the reactants must be broken to allow atoms to rearrange.
- New bonds must form between atoms to produce the products.

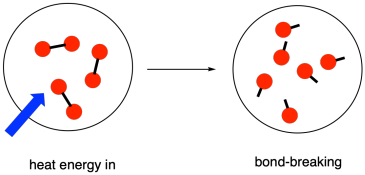
Bond Breaking is Endothermic
Breaking chemical bonds always requires an input of energy because it involves overcoming the electrostatic attraction between the bonded atoms. This energy is needed to separate atoms and pull them apart against the forces holding them together.
As a result, bond breaking is classified as an endothermic process, meaning energy is absorbed from the surroundings. The energy required to break a particular type of bond is called its bond enthalpy.
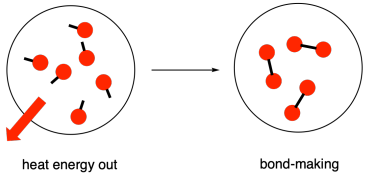
Bond Forming is Exothermic
Forming chemical bonds releases energy. When atoms bond, they move to a lower energy state and become more stable. The energy released corresponds to the strength of the new bond formed. This process is exothermic, as energy is given out to the surroundings.
The greater the bond strength, the more energy is released during bond formation.
Energy Profile Diagram
Energy profile diagrams visually represent the energy changes that occur during a chemical reaction:
- For an exothermic reaction, the products have a lower energy than the reactants. Energy is released.
- For an endothermic reaction, the products have a higher energy than the reactants. Energy is absorbed.
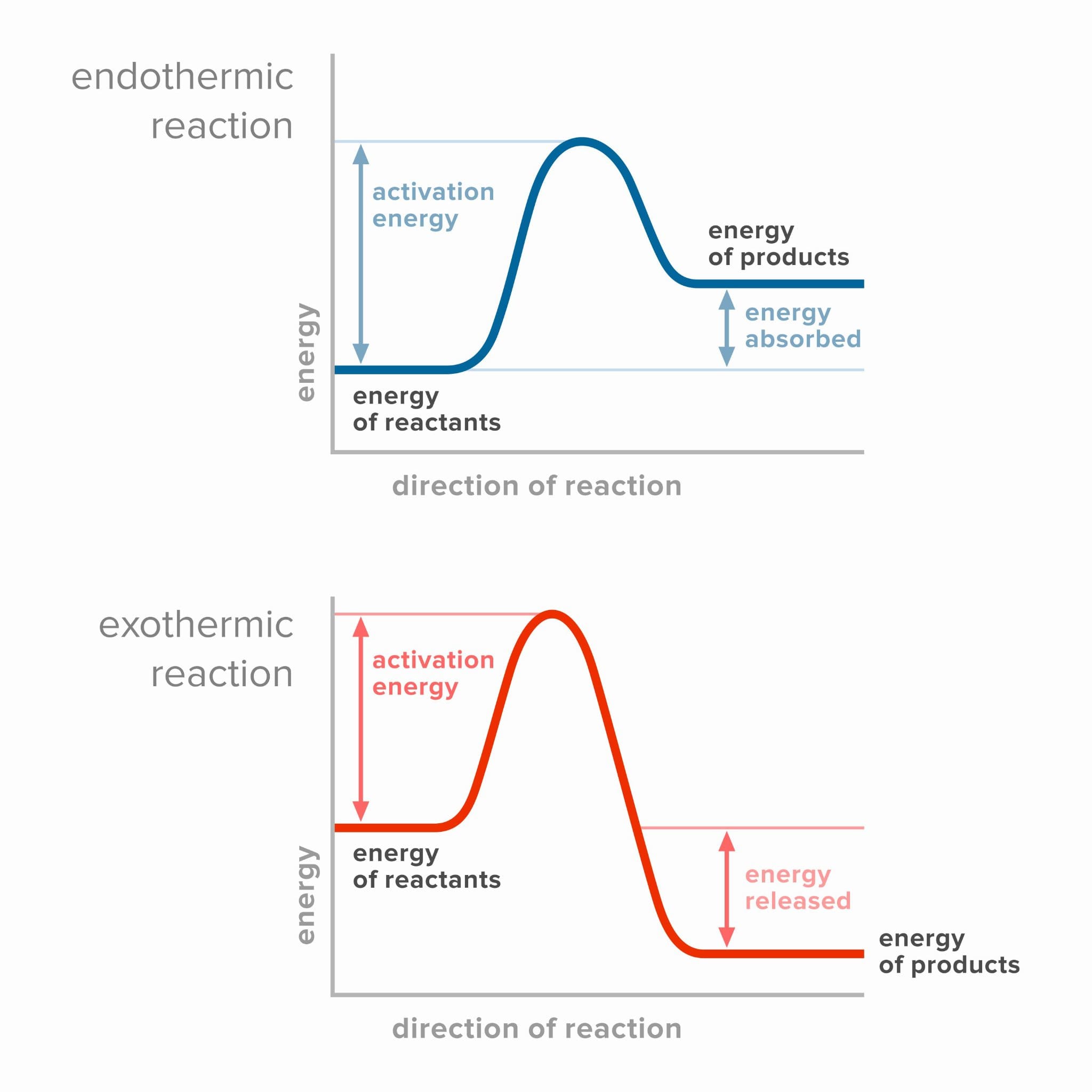
Both types of reactions require an initial input of energy known as the activation energy, which is the minimum energy required to initiate bond breaking in the reactants.
Example
Explain in detail why bond breaking is an endothermic process, and bond forming is exothermic. Include reference to molecular stability and energy changes.
▶️Answer/Explanation
Bond breaking requires energy because it involves disrupting the attractive forces that hold atoms together in a molecule. This process moves the system to a higher energy state and is therefore endothermic. For example, to break a covalent bond, energy must be supplied to overcome the shared electron attraction between the bonded nuclei.
In contrast, bond formation releases energy because atoms form bonds to reach a more stable, lower-energy configuration. As the atoms come together and form a bond, potential energy is released into the surroundings, making the process exothermic. The stronger the bond formed, the more energy is released. This is why reactions that form stable products often release significant amounts of energy.
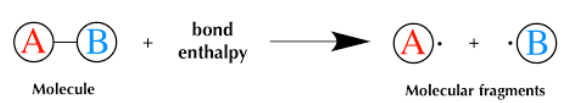
What Is Average Bond Enthalpy?
The average bond enthalpy is the average amount of energy required to break one mole of a specific type of covalent bond in the gaseous state. It is measured in kilojoules per mole (kJ/mol).
Because the same type of bond (e.g., O–H or C–H) can exist in different molecules with slightly different environments, the bond enthalpy value is averaged across many compounds to give a standardized value suitable for general use in calculations.
Key Formula for Enthalpy Change
The enthalpy change of a chemical reaction depends on the balance between energy absorbed to break bonds and energy released in forming new bonds. The overall enthalpy change of a reaction, \( \Delta H \), can be estimated using the following formula:
\( \Delta H = \sum \text{(Bond enthalpies of bonds broken)} – \sum \text{(Bond enthalpies of bonds formed)} \)
- Bonds broken: Energy absorbed (positive enthalpy change, endothermic)(\( \Delta H > 0 \))
- Bonds formed: Energy released (negative contribution to ΔH, exothermic)(\( \Delta H < 0 \))
Step-by-Step Calculation Method
- Write the balanced chemical equation for the reaction.
- List all bonds present in the reactants and products.
- Use average bond enthalpy values (from the data booklet) to calculate:
- Total energy required to break all bonds in the reactants
- Total energy released when new bonds are formed in the products
- Apply the formula:
\( \Delta H = \text{Total energy in bonds broken} – \text{Total energy in bonds formed} \)
Why Are These Values Averages?
The bond enthalpies are averaged values for the following reasons:
- The energy required to break a particular bond (e.g., C–H) can vary slightly depending on the surrounding atoms and molecular structure.
- For example, the O–H bond in water is slightly different from the O–H bond in ethanol or methanol.
- To provide a single usable value, data booklets report mean bond enthalpies averaged over a wide range of compounds.
Limitations of Using Average Bond Enthalpy Values
- They are valid only for substances in the gas phase. If the actual state is liquid or solid (e.g., liquid water), the enthalpy change will differ.
- They do not account for resonance structures (e.g., benzene), which distribute bonding over multiple atoms.
- They ignore intermolecular forces and energy changes associated with phase changes (e.g., vaporization or condensation).
- Because of these limitations, the ΔH calculated using bond enthalpies is only an approximation. Experimental values (e.g., from calorimetry) are usually more accurate.
Example
Calculate the approximate enthalpy change (\( \Delta H \)) for the complete combustion of methane:
\( \text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O} \)
Use the following average bond enthalpies (in kJ/mol):
C–H: 412, O=O: 498, C=O (in CO₂): 805, O–H: 463
▶️Answer/Explanation
Step 1: Bonds broken in the reactants
- CH₄ has 4 × C–H bonds: \( 4 \times 412 = 1648 \) kJ
- 2 O₂ molecules have 2 × O=O bonds: \( 2 \times 498 = 996 \) kJ
- Total energy absorbed (bonds broken) = 1648 + 996 = 2644 kJ
Step 2: Bonds formed in the products
- CO₂ has 2 × C=O bonds: \( 2 \times 805 = 1610 \) kJ
- 2 H₂O molecules have 4 × O–H bonds: \( 4 \times 463 = 1852 \) kJ
- Total energy released (bonds formed) = 1610 + 1852 = 3462 kJ
Step 3: Apply the formula
\( \Delta H = \text{Bonds broken} – \text{Bonds formed} \)
\( \Delta H = 2644 – 3462 = -818 \, \text{kJ/mol} \)
Conclusion: The combustion of methane is an exothermic reaction. Approximately 818 kJ of energy is released per mole of methane burned, based on average bond enthalpies.
Note: The actual experimental value may differ slightly because water is formed as a liquid in most real reactions, whereas bond enthalpy values assume the formation of gaseous water.
Example
Calculate the approximate enthalpy change (\( \Delta H \)) for the reaction between hydrogen and chlorine to form hydrogen chloride:
\( \text{H}_2 + \text{Cl}_2 \rightarrow 2\text{HCl} \)
Use the following average bond enthalpies (in kJ/mol):
H–H: 436, Cl–Cl: 243, H–Cl: 431
▶️Answer/Explanation
Step 1: Bonds broken (reactants)
- 1 × H–H bond = 436 kJ
- 1 × Cl–Cl bond = 243 kJ
- Total energy absorbed = 436 + 243 = 679 kJ
Step 2: Bonds formed (products)
- 2 × H–Cl bonds = \( 2 \times 431 = 862 \) kJ
- Total energy released = 862 kJ
Step 3: Calculate \( \Delta H \)
\( \Delta H = \text{Bonds broken} – \text{Bonds formed} \)
\( \Delta H = 679 – 862 = -183 \, \text{kJ/mol} \)
Conclusion: The reaction is exothermic, releasing approximately 183 kJ of energy per mole of hydrogen reacting with chlorine to form hydrogen chloride gas.
