Energy cycles in reactions: R1.2.3 Standard enthalpy changes of combustion and formation IB DP Chemistry Study Notes - New Syllabus 2025
Energy cycles in reactions – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 1.2.3 – Standard Enthalpy Changes of Combustion and Formation
Reactivity 1.2.3 – Standard Enthalpy Changes of Combustion and Formation
What Are Standard Enthalpy Changes?
Standard enthalpy changes represent the heat energy change that occurs during a chemical process under specific, defined conditions. These are:
- Pressure: 100 kPa (1 atmosphere)
- Temperature: 298 K (25 °C)
- Concentration: 1 mol dm⁻³ for all solutions
- All substances must be in their standard states (most stable physical form at 298 K and 100 kPa)
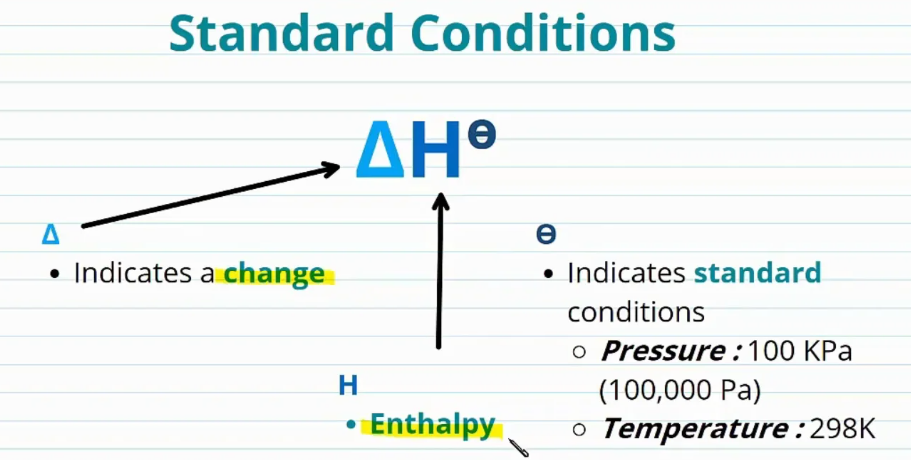
The standard enthalpy change is represented with the symbol \( \Delta H^\circ \), where the superscript \( \circ \) indicates standard conditions.
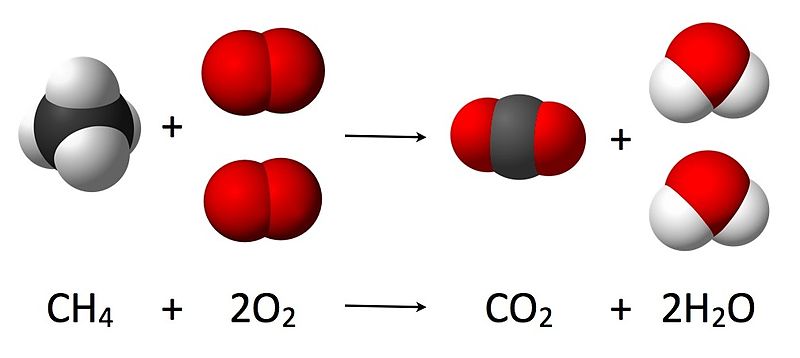
1. Standard Enthalpy Change of Combustion (\( \Delta H_c^\circ \))
This is defined as the enthalpy change when one mole of a substance undergoes complete combustion in excess oxygen under standard conditions.
\( \Delta H_c^\circ = \text{Enthalpy change when 1 mol of substance is completely burned in O}_2 \)
Key Features:
- The products of combustion are typically CO₂(g) and H₂O(l).
- Combustion reactions are always exothermic, so the enthalpy values are negative.
- The enthalpy change depends on the amount of substance combusted (always 1 mol for standard definition).
Example:
\( \text{CH}_4(g) + 2\text{O}_2(g) \rightarrow \text{CO}_2(g) + 2\text{H}_2\text{O}(l), \quad \Delta H_c^\circ = -890 \, \text{kJ/mol} \)
Here, -890 kJ of heat is released when 1 mole of methane undergoes complete combustion.
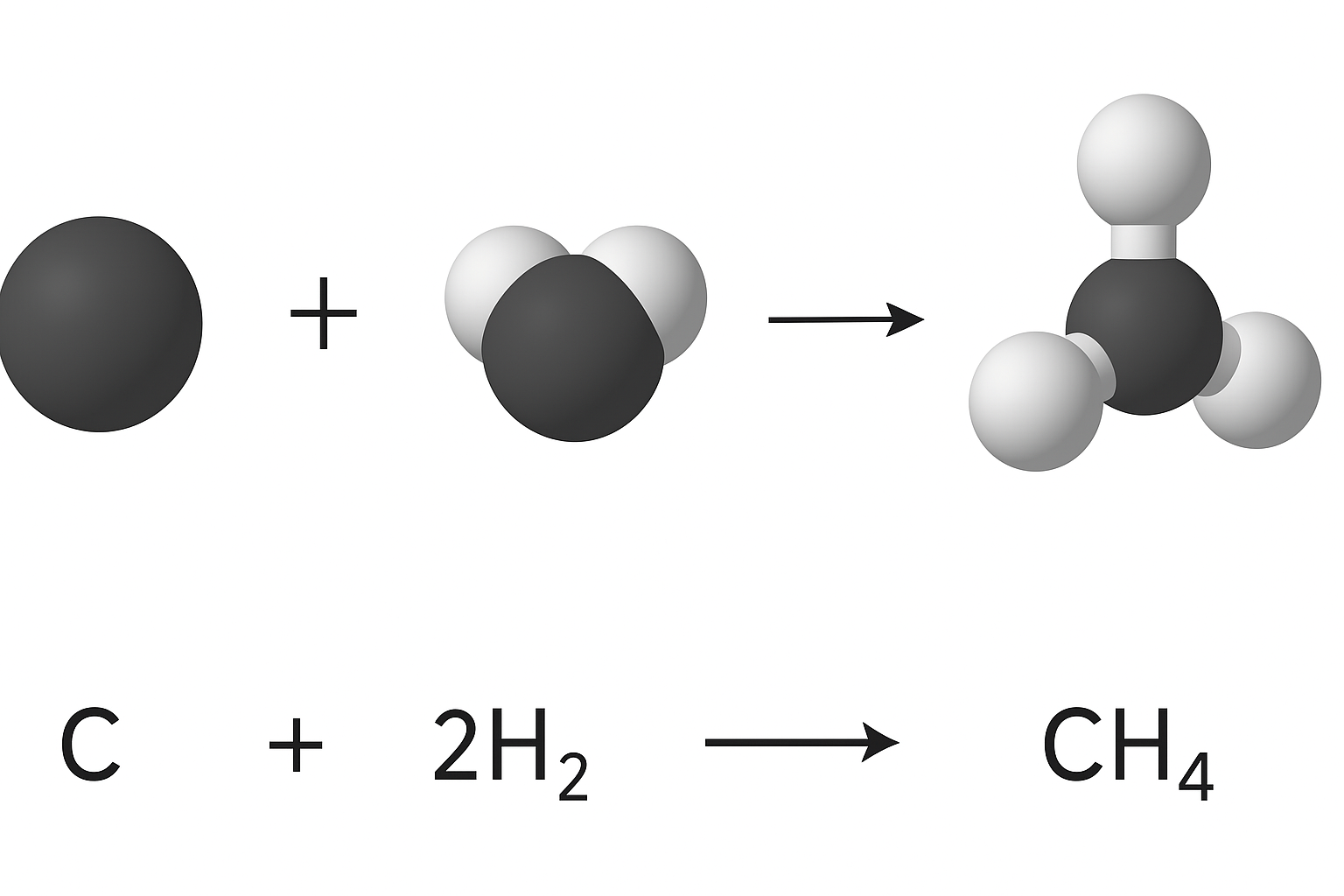
2. Standard Enthalpy Change of Formation (\( \Delta H_f^\circ \))
This is defined as the enthalpy change when 1 mole of a compound is formed from its elements in their standard states under standard conditions.
\( \Delta H_f^\circ = \text{Enthalpy change for forming 1 mol of compound from its elements in standard states} \)
Key Features:
- The equation must produce exactly 1 mole of the compound as the product.
- All reactants must be elements in their standard states.
- The sign of \( \Delta H_f^\circ \) may be positive (endothermic) or negative (exothermic), depending on the compound formed.
Example:
\( \text{C}(s) + 2\text{H}_2(g) \rightarrow \text{CH}_4(g), \quad \Delta H_f^\circ = -75 \, \text{kJ/mol} \)
This means that 75 kJ of energy is released when 1 mole of methane is formed from carbon and hydrogen in their standard states.
3. Importance in Thermodynamic Calculations
Both \( \Delta H_c^\circ \) and \( \Delta H_f^\circ \) values are widely used to determine enthalpy changes of other reactions using Hess’s Law. Since many reactions cannot be measured directly, these standard enthalpies allow indirect calculation of:
- Reaction enthalpies
- Formation and combustion of substances
- Energy comparisons between different chemical pathways
Calculating Enthalpy Changes Using \( \Delta H_f^\circ \) and \( \Delta H_c^\circ \)
1. Calculating Reaction Enthalpy Using Standard Enthalpies of Formation (\( \Delta H_f^\circ \))
To calculate the standard enthalpy change of a reaction using formation data, use the following equation:
\( \Delta H_{\text{reaction}}^\circ = \sum \Delta H_f^\circ(\text{products}) – \sum \Delta H_f^\circ(\text{reactants}) \)
Steps:
- Write the balanced chemical equation.
- Ensure all substances are in their standard states.
- Obtain \( \Delta H_f^\circ \) values for each compound.
- Multiply each \( \Delta H_f^\circ \) value by the number of moles in the equation.
- Apply the formula to calculate \( \Delta H_{\text{reaction}}^\circ \).
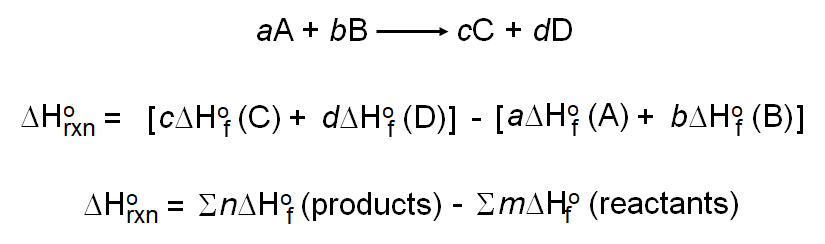
Example
Calculate the enthalpy change for the combustion of carbon monoxide:
\( 2\text{CO}(g) + \text{O}_2(g) \rightarrow 2\text{CO}_2(g) \)
Given:
- \( \Delta H_f^\circ(\text{CO}(g)) = -111 \, \text{kJ/mol} \)
- \( \Delta H_f^\circ(\text{CO}_2(g)) = -393.5 \, \text{kJ/mol} \)
- \( \Delta H_f^\circ(\text{O}_2(g)) = 0 \, \text{kJ/mol} \) (element in standard state)
▶️Answer/Explanation
Step 1: Apply the formula:
\( \Delta H^\circ = \left[2 \times (-393.5)\right] – \left[2 \times (-111) + 0\right] \)
\( = (-787.0) – (-222.0) = -565.0 \, \text{kJ} \)
Final Answer: \( \Delta H^\circ = -565.0 \, \text{kJ} \)
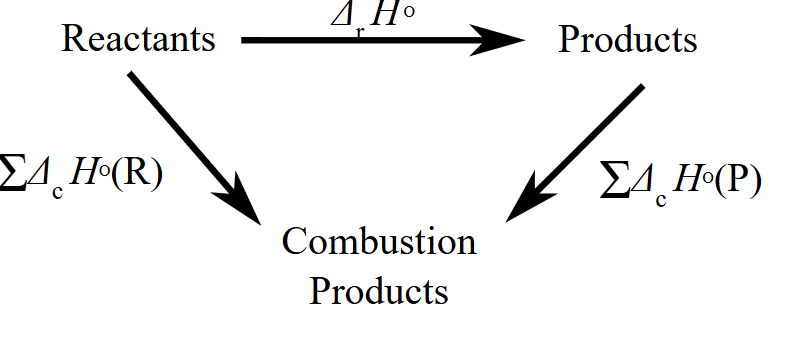
2. Calculating Reaction Enthalpy Using Standard Enthalpies of Combustion (\( \Delta H_c^\circ \))
When using combustion data, you must construct a Hess cycle. The following formula applies:
\( \Delta H_{\text{reaction}}^\circ = \sum \Delta H_c^\circ(\text{reactants}) – \sum \Delta H_c^\circ(\text{products}) \)
Why the formula is reversed: Combustion always leads to common products (CO₂ and H₂O), so we’re calculating the difference in energy released by burning the reactants vs. the products.
Steps:
- Write a balanced equation for the reaction.
- List the combustion enthalpies of each substance.
- Multiply each \( \Delta H_c^\circ \) by the number of moles involved.
- Apply the formula carefully (reactants minus products).
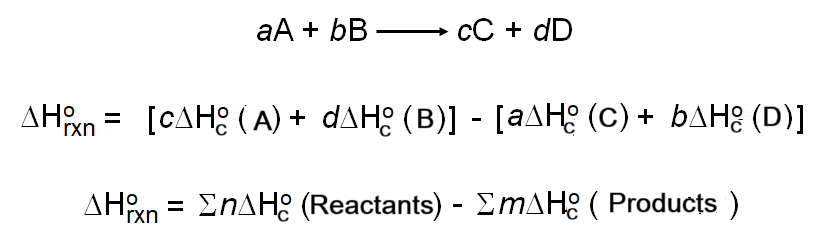
Example
Use combustion data to calculate the enthalpy of formation of ethanol:
\( 2\text{C}(s) + 3\text{H}_2(g) + \frac{1}{2}\text{O}_2(g) \rightarrow \text{C}_2\text{H}_5\text{OH}(l) \)
Given:
- \( \Delta H_c^\circ(\text{C}) = -393.5 \, \text{kJ/mol} \)
- \( \Delta H_c^\circ(\text{H}_2) = -285.8 \, \text{kJ/mol} \)
- \( \Delta H_c^\circ(\text{C}_2\text{H}_5\text{OH}) = -1367 \, \text{kJ/mol} \)
▶️Answer/Explanation
Step 1: Calculate total combustion enthalpy of the reactants:
- 2 × C: \( 2 \times -393.5 = -787.0 \, \text{kJ} \)
- 3 × H₂: \( 3 \times -285.8 = -857.4 \, \text{kJ} \)
- Total = \( -787.0 + (-857.4) = -1644.4 \, \text{kJ} \)
Step 2: Use the Hess cycle:
\( \Delta H_{\text{formation}}^\circ = \Delta H_c^\circ(\text{reactants}) – \Delta H_c^\circ(\text{product}) \)
\( = -1644.4 – (-1367) = -277.4 \, \text{kJ/mol} \)
Final Answer: \( \Delta H^\circ = -277.4 \, \text{kJ/mol} \)
Example
Use standard enthalpies of formation to calculate the enthalpy change for the reaction:
\( 3\text{C}(s) + 4\text{H}_2(g) \rightarrow \text{C}_3\text{H}_8(g) \)
Given data from the IB Data Booklet:
- \( \Delta H_f^\circ(\text{C}_3\text{H}_8(g)) = -104 \, \text{kJ/mol} \)
- \( \Delta H_f^\circ(\text{C}(s)) = 0 \, \text{kJ/mol} \)
- \( \Delta H_f^\circ(\text{H}_2(g)) = 0 \, \text{kJ/mol} \)
▶️Answer/Explanation
Step 1: Use the formula:
\( \Delta H_{\text{rxn}}^\circ = \sum \Delta H_f^\circ(\text{products}) – \sum \Delta H_f^\circ(\text{reactants}) \)
Step 2: Apply the values:
\( \Delta H^\circ = [-104] – [3 \times 0 + 4 \times 0] = -104 \, \text{kJ/mol} \)
Final Answer: \( \Delta H^\circ = -104 \, \text{kJ/mol} \)
This confirms that propane is exothermic in formation from its elements under standard conditions.
Key Tips and Common Pitfalls:
- Always double-check that the chemical equation is balanced before starting calculations.
- Be sure to use correct physical states (e.g., \( \text{H}_2\text{O}(l) \) vs. \( \text{H}_2\text{O}(g) \)) as values differ.
- Use data from the IB data booklet and verify the sign (enthalpies of combustion are always negative).
- Clearly distinguish between when you are using formation data (products – reactants) vs. combustion data (reactants – products).
