Energy from fuels: R1.3.4 Biofuels IB DP Chemistry Study Notes - New Syllabus 2025
Energy from fuels – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 1.3.4 — Biofuels
Reactivity 1.3.4 — Biofuels
Biofuels are fuels derived from recently living organisms, typically plants. They are considered renewable because they are produced by the short-term biological fixation of atmospheric carbon dioxide through photosynthesis.
What Are Biofuels?
- Biofuels are organic fuels made from biomass—recently living biological material such as crops, algae, or waste.
- Common examples include bioethanol (from fermentation of sugars), biodiesel (from vegetable oils), and biogas (from decomposition of organic waste).
- They are used as alternatives to fossil fuels in transportation and electricity generation.
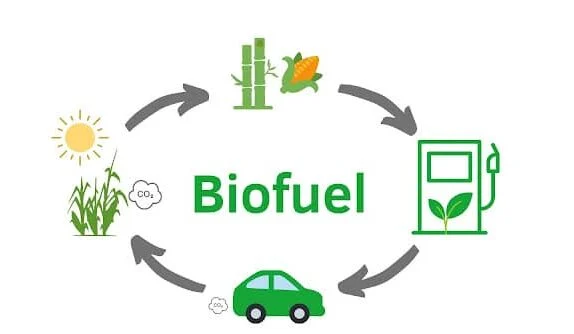
Photosynthesis: The Basis of Biofuel Production
Plants capture solar energy to convert atmospheric carbon dioxide and water into glucose, which can later be processed into fuels. Photosynthesis fixes atmospheric carbon into organic matter, providing the raw material for biofuel production.
Reaction:
\[ 6\text{CO}_2(g) + 6\text{H}_2\text{O}(l) \xrightarrow{\text{light energy}} \text{C}_6\text{H}_{12}\text{O}_6(aq) + 6\text{O}_2(g) \]
- CO₂: From the atmosphere
- H₂O: Absorbed from soil
- Light: Provides the energy required to drive the reaction (absorbed by chlorophyll)
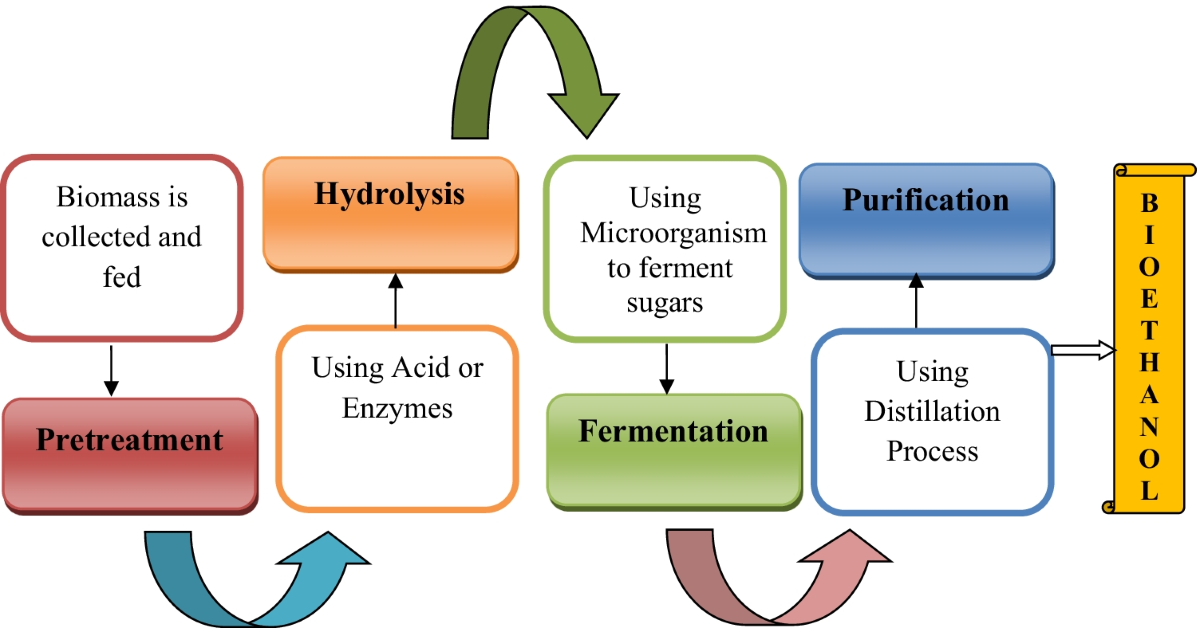
Conversion to Biofuels
- Bioethanol:
This is an alcohol-based fuel commonly blended with petrol. It is produced through the fermentation of plant-derived sugars (e.g., glucose from sugarcane or corn starch).
Reaction:
\[ \text{C}_6\text{H}_{12}\text{O}_6 \xrightarrow{\text{yeast}} 2\text{C}_2\text{H}_5\text{OH} + 2\text{CO}_2 \]
This fermentation process uses enzymes in yeast to break down glucose into ethanol and carbon dioxide. The ethanol is then distilled and dehydrated to create a fuel-grade product.
Advantages: Renewable, can be used in existing petrol engines, low sulfur emissions.
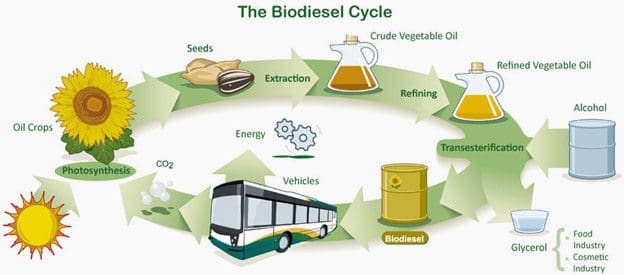
- Biodiesel:
Biodiesel is produced by transesterification, a chemical reaction where vegetable oils or animal fats react with alcohol (usually methanol) in the presence of a catalyst (e.g., NaOH or KOH) to form methyl esters (biodiesel) and glycerol.
General reaction:
\[ \text{Triglyceride} + 3\text{CH}_3\text{OH} \rightarrow 3\text{Fatty Acid Methyl Esters (FAME)} + \text{Glycerol} \]
The methyl esters are separated and purified for use in diesel engines. Biodiesel burns more cleanly than fossil diesel, with reduced particulate matter and CO emissions.
Advantages: Biodegradable, non-toxic, compatible with diesel engines, reduces dependency on petroleum.
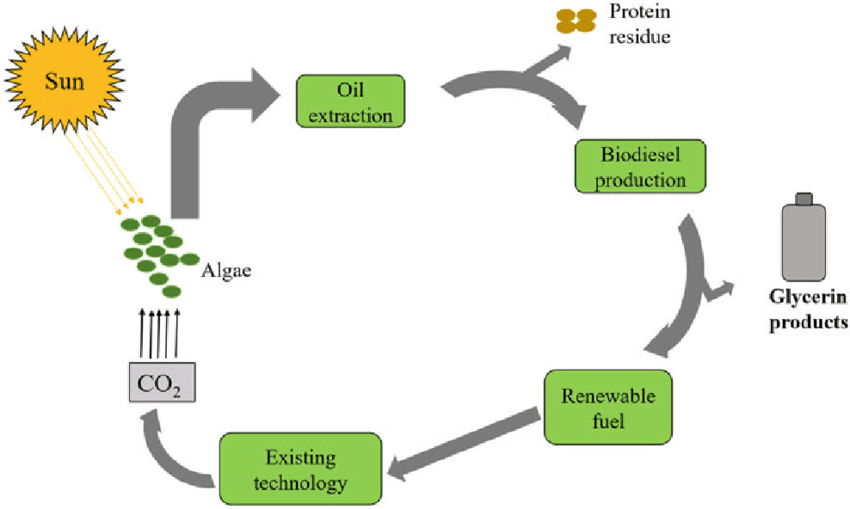
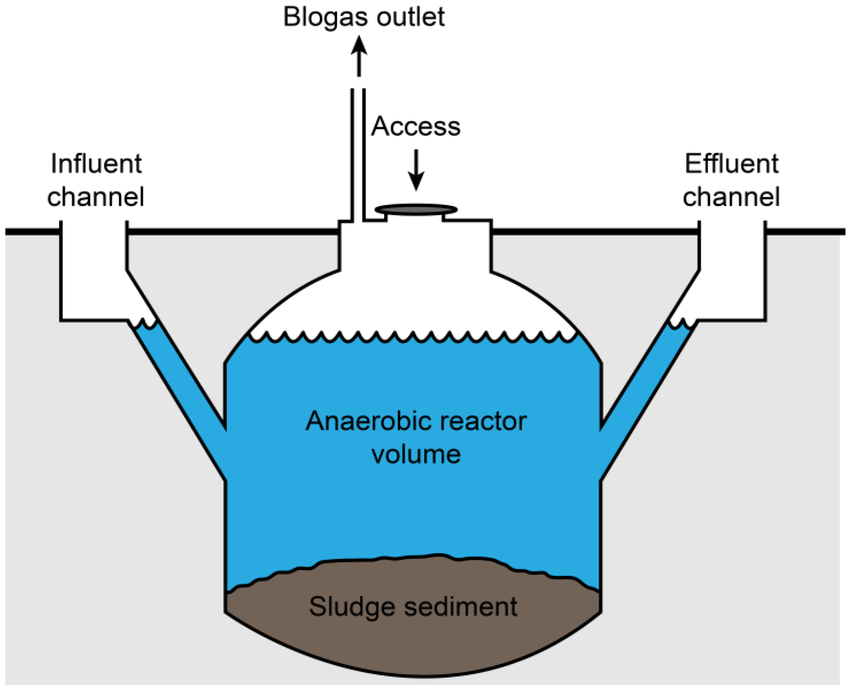
- Biogas:
Biogas is a mixture of gases (primarily methane and carbon dioxide) produced via anaerobic digestion of organic waste by bacteria in oxygen-free conditions. Common feedstocks include animal manure, food waste, and sewage sludge.
General process: Organic matter → digestion by bacteria → CH₄ + CO₂ + trace gases
\[ \text{C}_6\text{H}_{12}\text{O}_6 \xrightarrow{\text{anaerobic bacteria}} 3\text{CO}_2 + 3\text{CH}_4 \]
Biogas is captured and used for heating, electricity generation, or as a fuel for vehicles (after purification).
Advantages: Utilizes waste materials, reduces methane emissions from landfills, renewable source of energy.
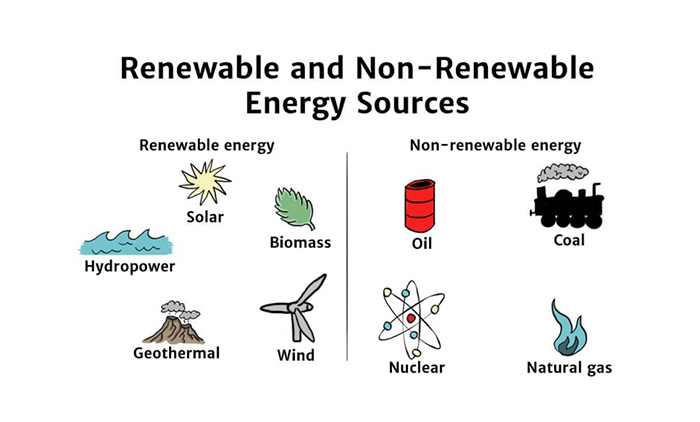
Renewable and Non-Renewable Energy Sources
| Aspect | Renewable | Non-Renewable |
|---|---|---|
| Availability | Replenished quickly | Finite and depleting |
| Environmental Impact | Low emissions, sustainable | High CO₂ and pollutant emissions |
| Cost | High initial investment, low operating cost | Often cheaper upfront, long-term cost varies |
| Examples | Bioethanol, wind, solar | Petrol, diesel, coal |
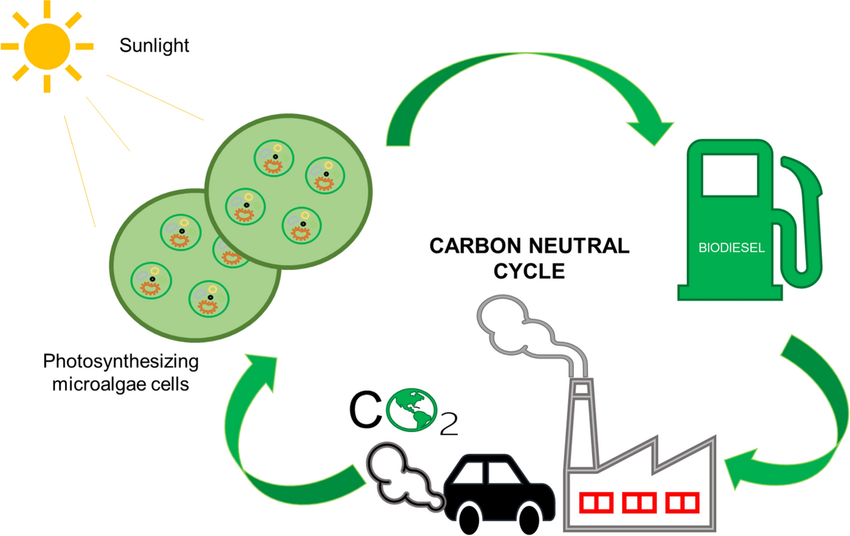
Why Biofuels Are Considered Renewable
- Carbon used to make the fuel comes from atmospheric CO₂, fixed during the plant’s lifetime.
- Combustion returns the same CO₂ back to the atmosphere — ideally forming a closed carbon cycle.
- Unlike fossil fuels, biofuels are regenerated on human timescales (months to years vs. millions of years).
Carbon Neutrality
Although biofuels emit CO₂ when burned, the idea is that the amount emitted is roughly equal to the amount taken in during photosynthesis. This is why biofuels are often referred to as carbon neutral, though in practice emissions from cultivation, processing, and transport must also be considered.
Advantages of Biofuels
- Produced from renewable sources (plants, organic waste)
- Help reduce net carbon emissions (part of short-term carbon cycle)
- Can often be used in existing engines and infrastructure
- Biodegradable and less toxic than fossil fuels
Disadvantages of Biofuels
- Lower energy density than fossil fuels
- Large-scale production may compete with food crops (e.g., corn)
- Land use change and deforestation can offset environmental benefits
- Requires energy for farming, harvesting, processing — not always carbon neutral
Environmental Considerations
Biofuels help reduce reliance on fossil fuels and contribute to climate goals, but their sustainability depends on:
- Land usage and biodiversity impacts
- Fertilizer and water use
- Lifecycle greenhouse gas emissions (from farm to fuel tank)
Example
Bioethanol and petrol are both used as transportation fuels. Despite the fact that combustion of bioethanol still releases carbon dioxide, it is often considered more environmentally friendly. Explain why, using a life-cycle analysis approach.
▶️Answer/Explanation
- Life-cycle analysis considers the total greenhouse gas emissions from raw material acquisition to fuel use.
- Bioethanol is derived from crops (e.g., sugarcane or corn) that absorb CO₂ during growth via photosynthesis:
\[ 6\text{CO}_2 + 6\text{H}_2\text{O} \rightarrow \text{C}_6\text{H}_{12}\text{O}_6 + 6\text{O}_2 \]
- When bioethanol is burned, the CO₂ released is roughly equal to that absorbed — creating a short-term carbon cycle:
\[ \text{C}_2\text{H}_5\text{OH} + 3\text{O}_2 \rightarrow 2\text{CO}_2 + 3\text{H}_2\text{O} \]
- In contrast, petrol is a fossil fuel derived from ancient biomass, and its combustion adds “new” CO₂ to the atmosphere that was locked away for millions of years:
\[ \text{C}_8\text{H}_{18} + \frac{25}{2} \text{O}_2 \rightarrow 8\text{CO}_2 + 9\text{H}_2\text{O} \]
- Therefore, bioethanol contributes less to net atmospheric CO₂, assuming sustainable crop management and minimal fossil input during farming, processing, and transport.
Example
Evaluate the environmental and ethical concerns of using large-scale agricultural land for biofuel production instead of food crops.
▶️Answer/Explanation
- Many first-generation biofuels (e.g., bioethanol from corn or sugarcane) require large areas of arable land.
- This can create competition with food production, driving up food prices and affecting food security, especially in developing nations.
- Large-scale monoculture biofuel farming can lead to deforestation, loss of biodiversity, and soil degradation — negating environmental benefits.
- Energy yield from biofuels is often lower per hectare compared to fossil fuels, meaning more land is needed for the same energy output.
- Second-generation biofuels (e.g., from agricultural waste or algae) are being developed to reduce these ethical and environmental trade-offs.
Conclusion: While biofuels offer renewable alternatives to fossil fuels, sustainable land use practices are essential to ensure they do not cause greater harm than benefit.
