Entropy and spontaneity: R1.4.1 Entropy (S) IB DP Chemistry Study Notes - New Syllabus 2025
Entropy and spontaneity – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 1.4.1 — Entropy \( (S) \)
Reactivity 1.4.1 — Entropy \( (S) \)
What is Entropy?
- Entropy (\( S \)) is a thermodynamic quantity representing the degree of disorder or randomness in a system. It is fundamentally linked to the number of ways energy and particles can be arranged at the microscopic level.
- The more disordered a system, the higher its entropy.
- Higher the entropy, higher the stability of the system.
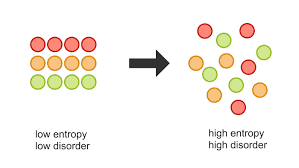
Key Characteristics of Entropy
- Symbol: \( S \); standard entropy = \( S^\circ \)
- Unit: \( \text{J K}^{-1} \text{mol}^{-1} \)
- State function: Depends only on the current state, not the path taken
- Third Law of Thermodynamics: \( S = 0 \) at 0 K for a perfect crystal
Endothermic Reactions and Entropy
- Endothermic reactions are chemical changes in which heat is absorbed from the surroundings, resulting in a positive enthalpy change (\( \Delta H > 0 \)). These reactions often feel cold to the touch.
- When a system absorbs energy during an endothermic reaction, particles may move more freely or energy may become more dispersed. This can lead to an increase in entropy (\( \Delta S > 0 \)).
- Endothermic processes often involve changes that increase disorder, such as melting, evaporation, or decomposition into gases.
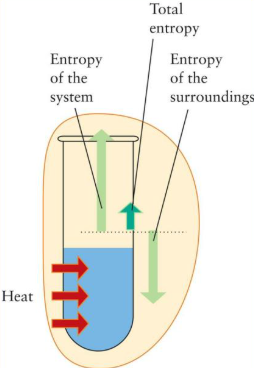
Examples of Endothermic Reactions with Increasing Entropy
- Dissolution of ammonium nitrate:
$ \text{NH}_4\text{NO}_3(s) \rightarrow \text{NH}_4^+(aq) + \text{NO}_3^-(aq) $ – Heat is absorbed from surroundings → endothermic
Entropy increases as solid dissociates into ions in solution - Thermal decomposition of calcium carbonate:
$ \text{CaCO}_3(s) \rightarrow \text{CaO}(s) + \text{CO}_2(g) $ – Requires heat input → endothermic
Entropy increases due to formation of gas (CO₂) - Evaporation of water:
$ \text{H}_2\text{O}(l) \rightarrow \text{H}_2\text{O}(g) $ – Endothermic: heat is needed for vaporization
Entropy increases: gas has more disorder than liquid
Relative Entropy of Physical States
Entropy depends on the physical state of a substance because of differences in particle freedom:
| State | Entropy | Explanation |
|---|---|---|
| Solid | Low | Particles vibrate in fixed positions , low randomness |
| Liquid | Moderate | Particles move more freely than solids |
| Gas | High | Particles are far apart and move randomly, highly disordered |
$ S_{\text{gas}} > S_{\text{liquid}} > S_{\text{solid}} $
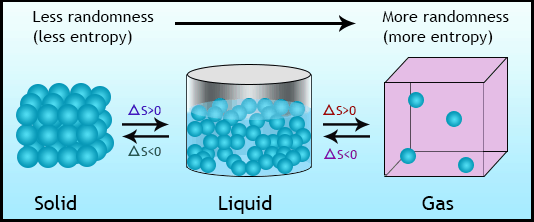
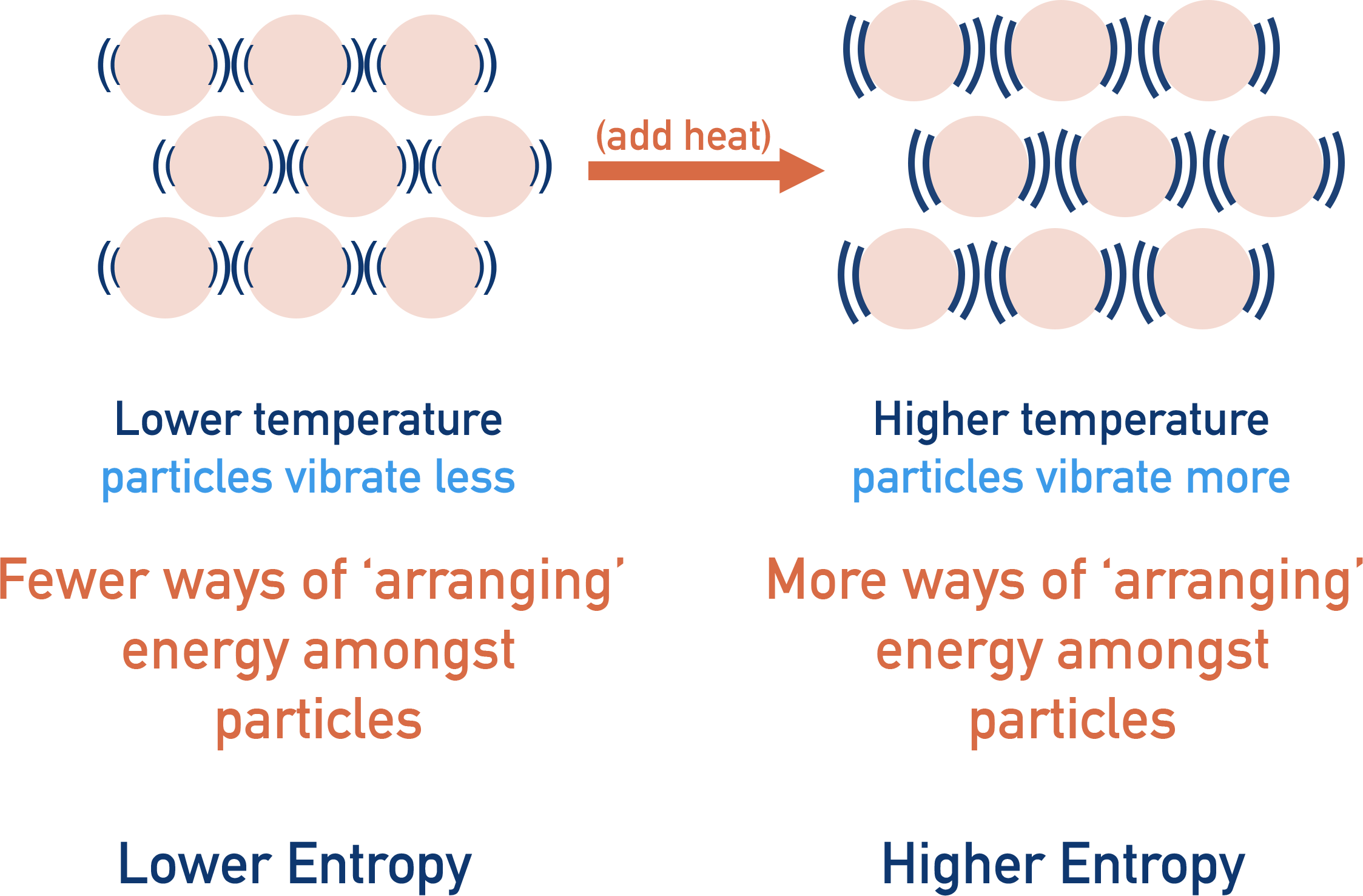
Factors Affecting Entropy
- Physical state: Gases have more entropy than liquids or solids.
- Temperature: Increasing temperature increases entropy.
- Number of particles: More moles of products (especially gases) = higher entropy.
- Mixing substances: Increases entropy due to higher dispersal of particles.
- Dissolution: Dissolving a solid into a solvent generally increases entropy.
Conceptual Examples
- Boiling water: Entropy increases (liquid → gas)
- Freezing water: Entropy decreases (liquid → solid)
- Combustion: Entropy usually increases (more gaseous products + energy release)
Entropy vs Enthalpy Comparison
| Property | Entropy (S) | Enthalpy (H) |
|---|---|---|
| Definition | Measure of disorder/randomness | Heat content of a system |
| Units | \( \text{J K}^{-1} \text{mol}^{-1} \) | \( \text{kJ mol}^{-1} \) |
| Symbol | S | H |
| Function Type | State function | State function |
| Effect on Spontaneity | Higher S favours spontaneity | Exothermic (\( \Delta H < 0 \)) favours spontaneity |
Why is Entropy Important?
- It plays a key role in determining whether a process is spontaneous.
- Used in the Gibbs free energy equation:
$ \Delta G = \Delta H – T \Delta S $
Entropy and Stability
The higher the entropy, the greater the number of possible microstates (ways to arrange the system), which contributes to greater thermodynamic stability.
Because systems naturally evolve towards states that are statistically more probable — those with higher entropy.
At equilibrium, a system tends to maximize its entropy while minimizing its free energy:
$ \Delta G = \Delta H – T\Delta S $
- If \( \Delta S \) is large and positive, it can drive \( \Delta G \) to be negative — making the process spontaneous and stable.
- This is especially true at higher temperatures where the \( T\Delta S \) term dominates.
- Nature tends toward increasing entropy (greater disorder).
Example
Predict the entropy change for the following processes:
- Melting of ice
- Condensation of steam
- Combustion of methane
▶️Answer/Explanation
- Melting of ice: Increase in entropy (solid → liquid)
- Condensation of steam: Decrease in entropy (gas → liquid)
- Combustion of methane: Increase in entropy (more gaseous products, energy released)
Predicting Entropy Changes in Physical and Chemical Processes
Entropy (\( S \)) changes can be predicted qualitatively based on how the distribution of matter or energy changes during a process. The key idea is: more disorder = higher entropy.
General Guidelines for Predicting Entropy Change (\( \Delta S \)):
- Change of state: Entropy increases when going from solid → liquid → gas.
- Increase in the number of gas molecules: Entropy increases if more moles of gas are formed.
- Dissolution: Dissolving a solid or gas in a solvent increases entropy.
- Temperature increase: Higher temperatures increase particle motion and entropy.
Examples:
- \( \text{H}_2\text{O}(s) \rightarrow \text{H}_2\text{O}(l) \): Entropy increases (melting)
- \( \text{CO}_2(g) \rightarrow \text{CO}_2(s) \): Entropy decreases (deposition)
- \( \text{N}_2(g) + 3\text{H}_2(g) \rightarrow 2\text{NH}_3(g) \): Entropy decreases (fewer gas particles)
Calculating Standard Entropy Change (\( \Delta S^\circ \))
The standard entropy change of a reaction is calculated using standard molar entropy values \( S^\circ \) (from the Data Booklet) using the formula:
$ \Delta S^\circ = \sum S^\circ_{\text{products}} – \sum S^\circ_{\text{reactants}} $
Where \( S^\circ \) values are in \( \text{J K}^{-1} \text{mol}^{-1} \).
Steps:
- Write a balanced chemical equation.
- Look up the standard entropy values (\( S^\circ \)) for each substance.
- Multiply each \( S^\circ \) by its coefficient in the equation.
- Subtract total reactants from total products.
Example
Calculate the standard entropy change \( \Delta S^\circ \) for the reaction:
$ \text{N}_2(g) + 3\text{H}_2(g) \rightarrow 2\text{NH}_3(g) $
Given standard molar entropies:
- \( S^\circ(\text{N}_2) = 192 \, \text{J K}^{-1} \text{mol}^{-1} \)
- \( S^\circ(\text{H}_2) = 131 \, \text{J K}^{-1} \text{mol}^{-1} \)
- \( S^\circ(\text{NH}_3) = 193 \, \text{J K}^{-1} \text{mol}^{-1} \)
▶️Answer/Explanation
Step 1: Multiply standard entropies by the number of moles:
\( \sum S^\circ_{\text{products}} = 2 \times 193 = 386 \)
\( \sum S^\circ_{\text{reactants}} = 192 + (3 \times 131) = 192 + 393 = 585 \)
Step 2: Calculate \( \Delta S^\circ \):
$ \Delta S^\circ = 386 – 585 = -199 \, \text{J K}^{-1} \text{mol}^{-1} $
Conclusion: Entropy decreases in this reaction, as gaseous molecules are being combined into fewer, more ordered molecules.
Example
Explain why the dissolution of ammonium nitrate in water is spontaneous even though it is endothermic.
▶️Answer/Explanation
Step 1: Reaction
$ \text{NH}_4\text{NO}_3(s) \rightarrow \text{NH}_4^+(aq) + \text{NO}_3^-(aq) $
Step 2: Enthalpy Change — The process is endothermic: \( \Delta H > 0 \)
Step 3: Entropy Change — Solid turns into solvated ions, greatly increasing disorder: \( \Delta S \gg 0 \)
Step 4: Gibbs Free Energy — Despite \( \Delta H > 0 \), the large \( T\Delta S \) term makes:
$ \Delta G = \Delta H – T\Delta S < 0 $
Conclusion: The increase in entropy is so significant that it outweighs the energy absorbed, making the process spontaneous and more stable thermodynamically.
Tips for IB Exams:
- Always check physical states — gases have the biggest impact on entropy.
- Be aware of units — entropy is often in J, while enthalpy is in kJ.
- Use the Data Booklet to find accurate \( S^\circ \) values.
