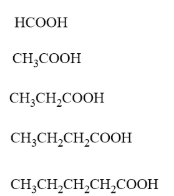Functional groups: S3.2.3 Homologous series IB DP Chemistry Study Notes - New Syllabus 2025
Functional groups: Classification of organic compounds – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Structure 3.2.3 – Homologous Series
Structure 3.2.3 – Homologous Series
A homologous series is a family of organic compounds with the same functional group and similar chemical properties. Members of a homologous series differ from each other by a constant structural unit, typically a methylene group \( (\text{CH}_2) \).
Characteristics of a Homologous Series
- All members contain the same functional group.
- Each successive compound differs by an additional \( \text{CH}_2 \) group (14 amu).
- They share the same general formula.
- They have similar chemical properties due to the common functional group.
- They exhibit gradual variation in physical properties (e.g., boiling point, solubility).
Why Homologous Series Are Important
- They help in systematically classifying organic compounds.
- They simplify the study of chemical properties by grouping similar compounds together.
- They assist in understanding trends in physical and chemical behavior across a group.
Major Homologous Series in Organic Chemistry
Below are the main homologous series required for IBDP Chemistry. Each series contains compounds with the same functional group and follows a general formula. Successive members differ by a –\( \text{CH}_2 \)– unit.
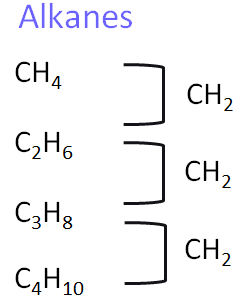
1. Alkanes
Alkanes are saturated hydrocarbons containing only single bonds between carbon atoms. They are relatively unreactive except in combustion or substitution reactions.
- Homologous series: Alkanes (saturated hydrocarbons)
- General formula: \( \text{C}_n\text{H}_{2n+2} \)
- Functional group: None (single C–C bonds only)
- First compound: Methane – \( \text{CH}_4 \)
- Next members: Ethane –\( \text{CH}_3\text{CH}_3 \), Propane – \( \text{CH}_3\text{CH}_2\text{CH}_3 \), Butane –\( \text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_3 \)

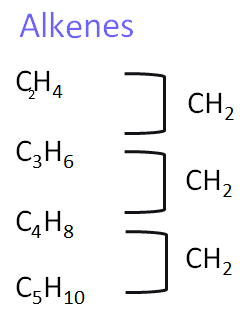
2. Alkenes
Alkenes are unsaturated hydrocarbons that contain at least one carbon–carbon double bond. They are more reactive than alkanes and undergo addition reactions.
- Homologous series: Alkenes (unsaturated hydrocarbons with C=C)
- General formula: \( \text{C}_n\text{H}_{2n} \)
- Functional group: C=C double bond
- First compound: Ethene – \( \text{CH}_2\text{CH}_2 \)
- Next members: Propene – \( \text{CH}_2\text{CHCH}_3 \), Butene – \( \text{CH}_2\text{CHCH}_2\text{CH}_3 \), Pentene – \( \text{CH}_2\text{CHCH}_2\text{CH}_2\text{CH}_3 \)

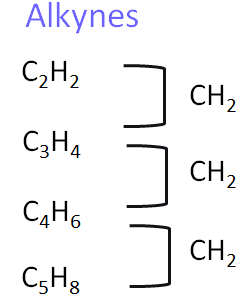
3. Alkynes
Alkynes are unsaturated hydrocarbons that contain at least one carbon–carbon triple bond. They undergo similar addition reactions to alkenes.
- Homologous series: Alkynes (unsaturated hydrocarbons with C≡C)
- General formula: \( \text{C}_n\text{H}_{2n-2} \)
- Functional group: C≡C triple bond
- First compound: Ethyne – \( \text{CH}\equiv\text{CH} \)
- Next members: Propyne – \( \text{CH}\equiv\text{CCH}_3 \), Butyne – \( \text{CH}_3\text{C}\equiv\text{CCH}_3 \), Pentyne – \( \text{CH}_3\text{CH}_2\text{C}\equiv\text{CH} \)

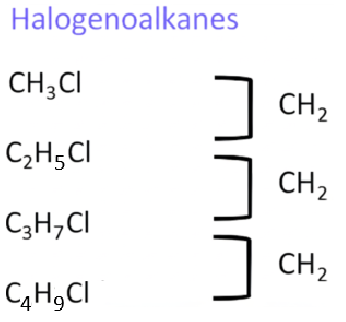
4. Halogenoalkanes
These are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogens (Cl, Br, or I). They undergo nucleophilic substitution reactions.
- Homologous series: Halogenoalkanes (alkyl halides)
- Functional group: –X (X = Cl, Br, I)
- First compound: Chloromethane – \( \text{CH}_3\text{Cl} \)
- Next members: Chloroethane – \( \text{CH}_3\text{CH}_2\text{Cl} \), 1-Chloropropane – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{Cl} \), 1-Chlorobutane – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2\text{Cl} \)

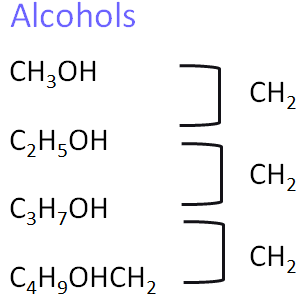
5. Alcohols
Alcohols contain the hydroxyl (–OH) functional group. They are polar and can hydrogen bond, making them soluble in water. They undergo combustion and oxidation.
- Homologous series: Alcohols
- General formula: \( \text{C}_n\text{H}_{2n+1}\text{OH} \)
- Functional group: –OH (hydroxyl group)
- First compound: Methanol – \( \text{CH}_3\text{OH} \)
- Next members: Ethanol – \( \text{CH}_3\text{CH}_2\text{OH} \), Propanol – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{OH} \), Butanol – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2\text{OH} \)

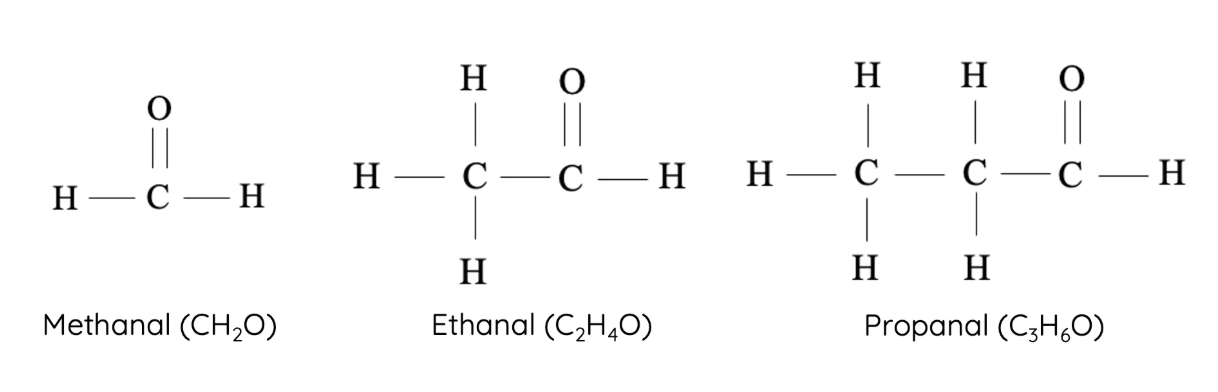
6. Aldehydes
Aldehydes have a carbonyl group (C=O) at the end of the carbon chain. They are produced by the partial oxidation of primary alcohols.
- Homologous series: Aldehydes
- Functional group: –CHO (carbonyl at the end)
- First compound: Methanal – \( \text{HCHO} \)
- Next members: Ethanal – \( \text{CH}_3\text{CHO} \), Propanal – \( \text{CH}_3\text{CH}_2\text{CHO} \), Butanal – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{CHO} \)

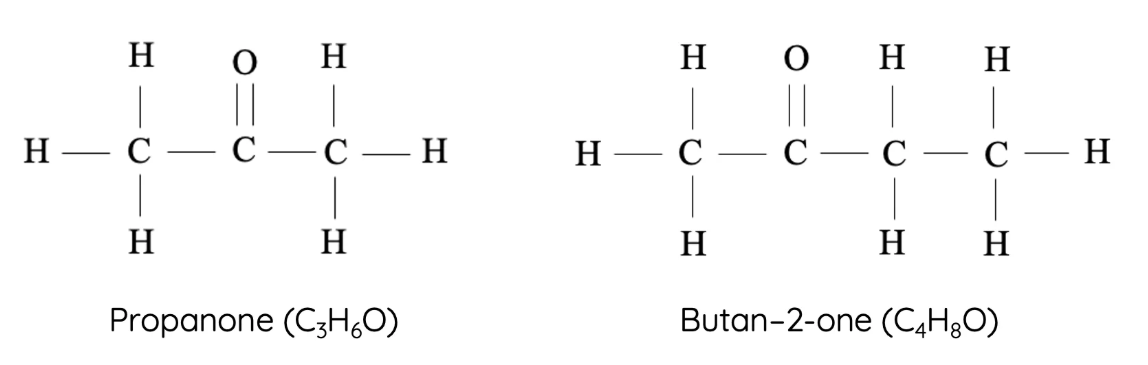
7. Ketones
Ketones contain a carbonyl group located within the carbon chain (not at the end). They are formed by oxidation of secondary alcohols.
- Homologous series: Ketones
- Functional group: –CO– (carbonyl in the middle)
- First compound: Propanone – \( \text{CH}_3\text{COCH}_3 \)
- Next members: Butanone – \( \text{CH}_3\text{COCH}_2\text{CH}_3 \), Pentanone – \( \text{CH}_3\text{COCH}_2\text{CH}_2\text{CH}_3 \), Hexanone – \( \text{CH}_3\text{COCH}_2\text{CH}_2\text{CH}_2\text{CH}_3 \)

8. Carboxylic Acids
These are organic acids containing the carboxyl (–COOH) group. They are weak acids and react with metals, carbonates, and bases.
- Homologous series: Carboxylic acids
- General formula: \( \text{C}_n\text{H}_{2n+1}\text{COOH} \)
- Functional group: –COOH (carboxyl group)
- First compound: Methanoic acid – \( \text{HCOOH} \)
- Next members: Ethanoic acid – \( \text{CH}_3\text{COOH} \), Propanoic acid – \( \text{CH}_3\text{CH}_2\text{COOH} \), Butanoic acid – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{COOH} \)

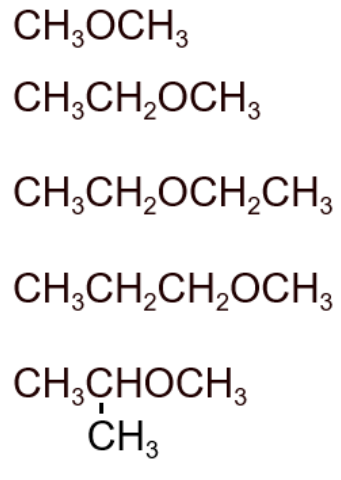
9. Ethers
Ethers contain an oxygen atom connected to two alkyl groups (R–O–R′). They are generally unreactive and used as solvents.
- Homologous series: Ethers
- Functional group: –O– (alkoxy group)
- First compound: Methoxymethane – \( \text{CH}_3\text{OCH}_3 \)
- Next members: Methoxyethane – \( \text{CH}_3\text{OCH}_2\text{CH}_3 \), Ethoxyethane – \( \text{CH}_3\text{CH}_2\text{OCH}_2\text{CH}_3 \), Propoxyethane – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{OCH}_2\text{CH}_3 \)

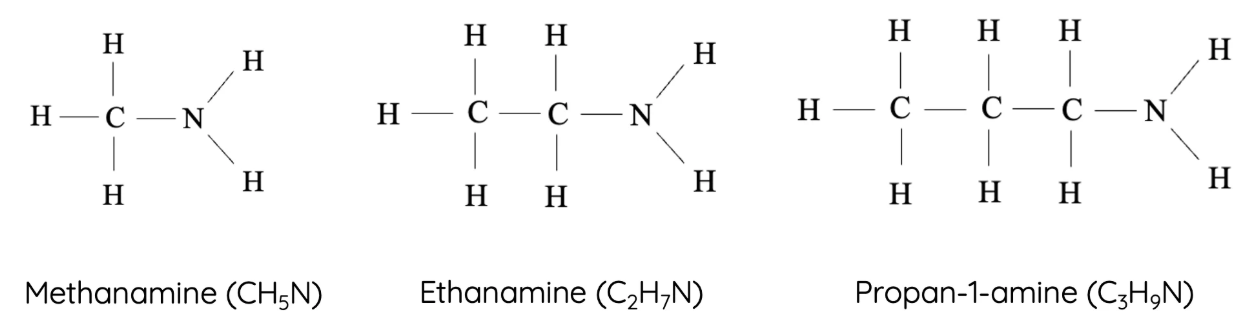
10. Amines
Amines contain a nitrogen atom bonded to carbon and/or hydrogen. They are basic and derived from ammonia.
- Homologous series: Primary amines
- Functional group: –NH₂ (amino group)
- First compound: Methanamine – \( \text{CH}_3\text{NH}_2 \)
- Next members: Ethanamine – \( \text{CH}_3\text{CH}_2\text{NH}_2 \), Propanamine – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{NH}_2 \), Butanamine – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2\text{NH}_2 \)

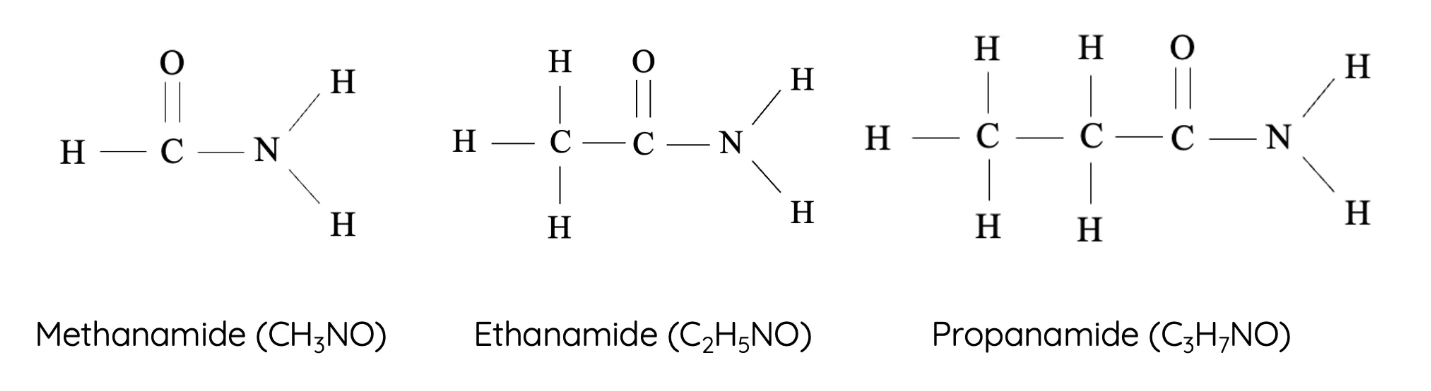
11. Amides
Amides contain both a carbonyl group and an amine group. They are formed from reactions between carboxylic acids and amines or ammonia.
- Homologous series: Amides
- Functional group: –CONH₂ (carboxamide group)
- First compound: Methanamide – \( \text{HCONH}_2 \)
- Next members: Ethanamide – \( \text{CH}_3\text{CONH}_2 \), Propanamide – \( \text{CH}_3\text{CH}_2\text{CONH}_2 \), Butanamide – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{CONH}_2 \)

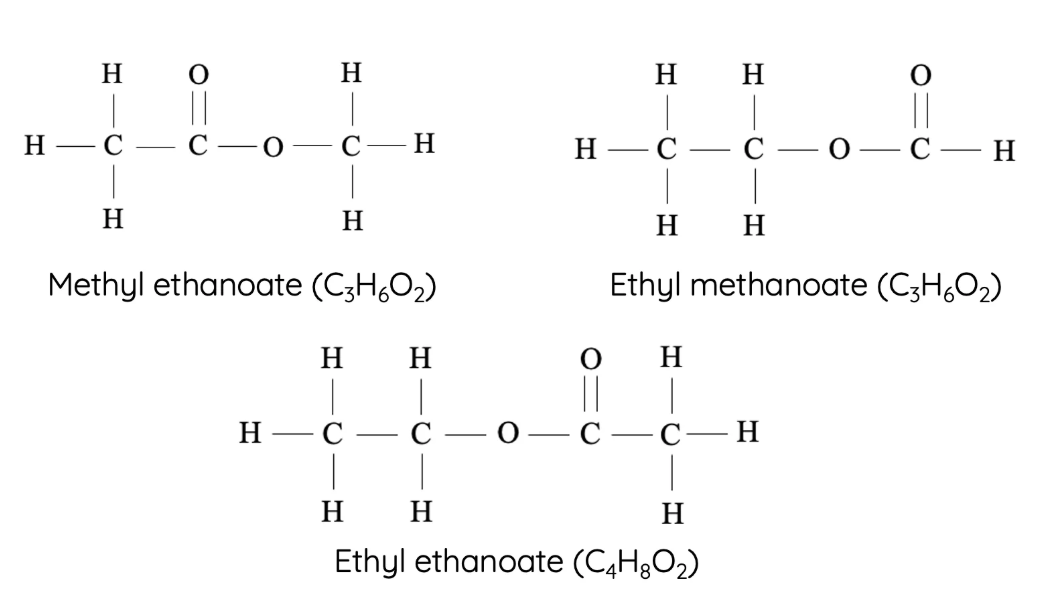
12. Esters
Esters are formed from the reaction between a carboxylic acid and an alcohol. They are known for their sweet/fruity smells and are used in perfumes and food flavoring.
- Homologous series: Esters
- Functional group: –COO– (ester linkage)
- First compound: Methyl methanoate – \( \text{HCOOCH}_3 \)
- Next members: Methyl ethanoate – \( \text{CH}_3\text{COOCH}_3 \), Ethyl ethanoate – \( \text{CH}_3\text{COOCH}_2\text{CH}_3 \), Propyl ethanoate – \( \text{CH}_3\text{COOCH}_2\text{CH}_2\text{CH}_3 \)

Example
List the first three members of the carboxylic acid homologous series. Explain how they are related.
▶️Answer/Explanation
- Methanoic acid: \( \text{HCOOH} \)
- Ethanoic acid: \( \text{CH}_3\text{COOH} \)
- Propanoic acid: \( \text{CH}_3\text{CH}_2\text{COOH} \)
Each compound differs by one \( \text{CH}_2 \) group.
All contain the carboxyl group (–COOH), hence belong to the same homologous series.
Example
List the first three members of the alcohol homologous series and explain how they differ structurally.
▶️Answer/Explanation
Alcohols have the general formula: \( \text{C}_n\text{H}_{2n+1}\text{OH} \)
First three members:
- Methanol – \( \text{CH}_3\text{OH} \)
- Ethanol – \( \text{CH}_3\text{CH}_2\text{OH} \)
- Propanol – \( \text{CH}_3\text{CH}_2\text{CH}_2\text{OH} \)
Each successive member differs by a –\( \text{CH}_2 \)– unit.
All contain the hydroxyl functional group (–OH), hence show similar chemical reactions.
Example
A compound has the molecular formula \( \text{C}_4\text{H}_8\text{O}_2 \). It is a liquid at room temperature, has a sweet smell, and does not react with sodium carbonate.
Another compound with formula \( \text{C}_3\text{H}_6\text{O}_2 \) shows similar properties.
Identify the homologous series these compounds belong to. Justify your answer and suggest a possible structure for the \( \text{C}_4\text{H}_8\text{O}_2 \) compound.
▶️Answer/Explanation
The compounds have the general formula of esters: \( \text{C}_n\text{H}_{2n}\text{O}_2 \). Esters are commonly known for having a sweet or fruity odor.
The fact that they do not react with sodium carbonate suggests they are not carboxylic acids (which would effervesce due to CO₂ release).
Both compounds are likely esters, which belong to the ester homologous series.
A possible structure for \( \text{C}_4\text{H}_8\text{O}_2 \) is methyl propanoate: \( \text{CH}_3\text{CH}_2\text{COOCH}_3 \)
The second compound, \( \text{C}_3\text{H}_6\text{O}_2 \), could be methyl ethanoate: \( \text{CH}_3\text{COOCH}_3 \)
Therefore, both belong to the ester homologous series, differing by one –\( \text{CH}_2 \)– unit.