Functional groups: S3.2.4 Physical trends in homologous series IB DP Chemistry Study Notes - New Syllabus 2025
Functional groups: Classification of organic compounds – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Structure 3.2.4 – Trends in Physical Properties in a Homologous Series
Structure 3.2.4 – Trends in Physical Properties in a Homologous Series
In a homologous series, each compound differs from the next by a –\( \text{CH}_2 \)– unit, but they share the same functional group. This consistent structural difference results in gradual trends in physical properties such as boiling point, melting point, solubility, and density.
Boiling Point
The boiling point increases as the number of carbon atoms in the chain increases. This is primarily due to:
- Greater molecular mass: More electrons → stronger temporary dipoles → stronger London dispersion forces.
- Larger surface area: Longer chains increase contact points between molecules → stronger intermolecular attractions.
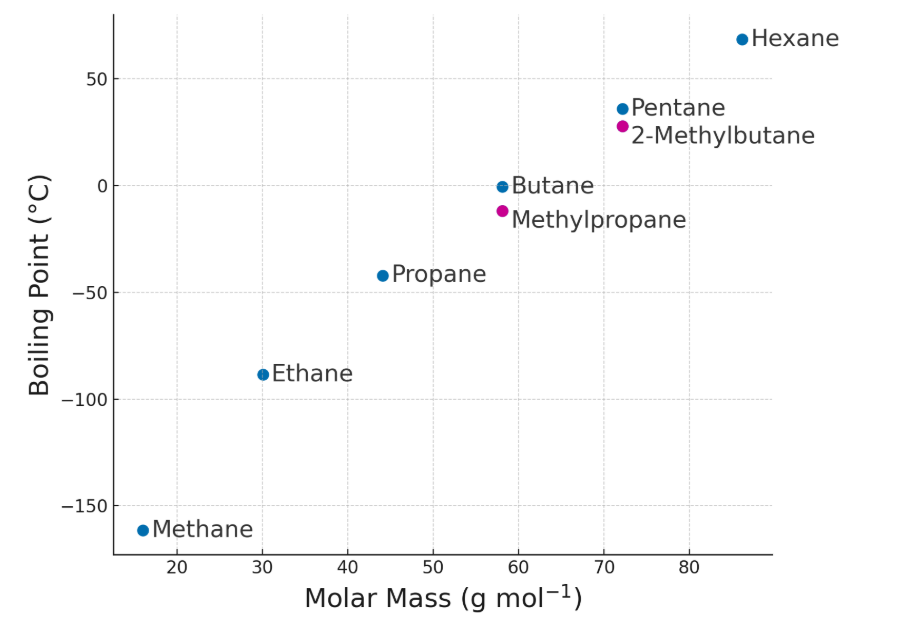
Result: More energy is needed to overcome intermolecular forces, leading to higher boiling points for larger molecules.
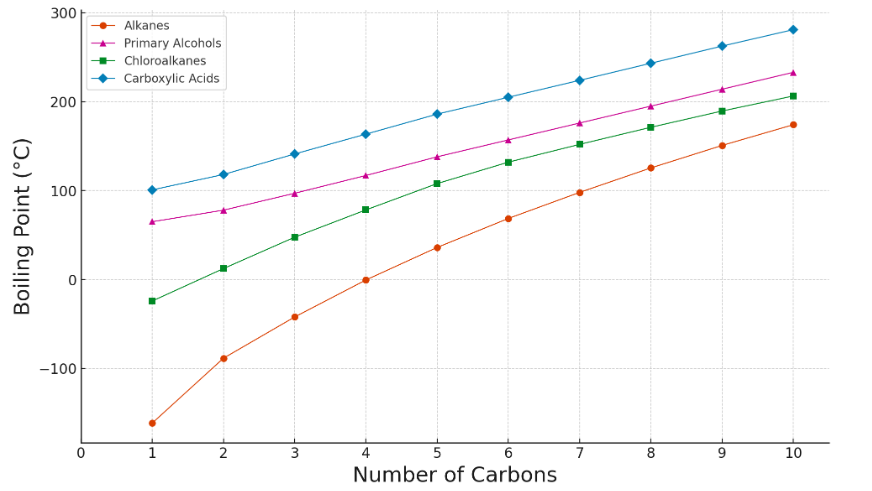
Melting Point
Melting points also generally increase with increasing chain length but less smoothly than boiling points. The irregular trend is due to:
- Packing efficiency: Even-numbered alkanes pack more tightly in the solid state than odd-numbered ones.
- Molecular symmetry: Affects how easily molecules form a crystal lattice.
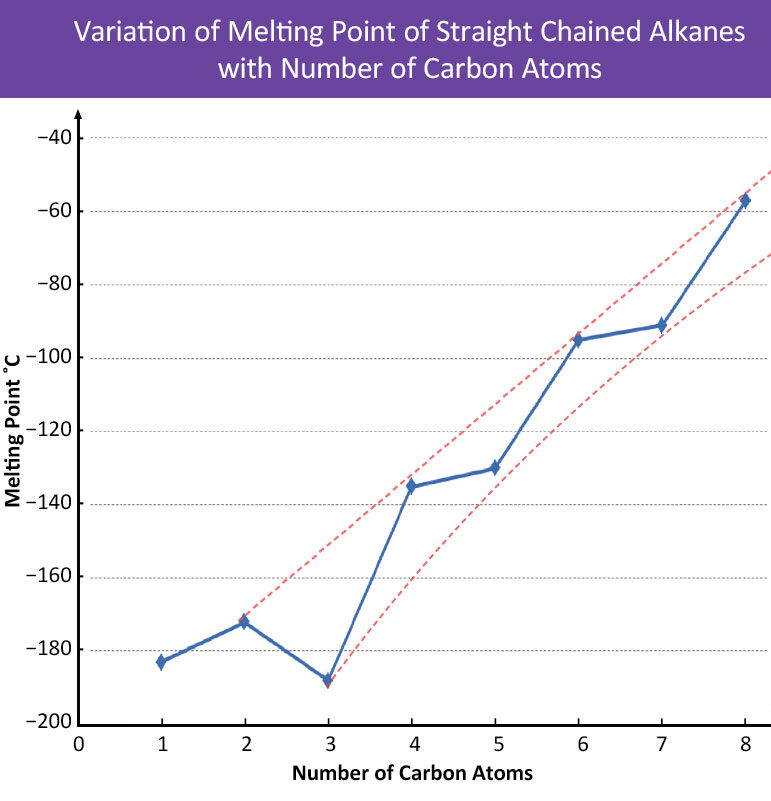
Additional Trends
Solubility (in Water)
- For polar compounds (like alcohols, acids): short chains are soluble due to hydrogen bonding.
- As the non-polar hydrocarbon chain grows, solubility decreases due to reduced polarity.
- Nonpolar compounds (alkanes, alkenes) are generally insoluble in water regardless of chain length.
Volatility
Volatility (tendency to vaporize) decreases with increasing molecular size due to stronger intermolecular forces.
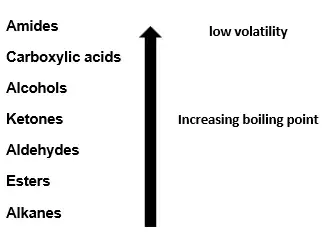
Density
Density shows a gradual increase in liquids and solids due to increased molar mass and closer molecular packing.
Example
Compare the boiling points of methane, ethane, propane, and butane. Explain the trend based on their position in the alkane homologous series.
▶️Answer/Explanation
Boiling points:
- Methane: –161°C
- Ethane: –89°C
- Propane: –42°C
- Butane: –0.5°C
Each compound differs by one –\( \text{CH}_2 \)– group, increasing molar mass and size.
Increased London dispersion forces lead to higher boiling points.
Example
Compare the solubility of methanol, ethanol, and butanol in water.
▶️Answer/Explanation
Methanol and ethanol are highly soluble in water due to their –OH group forming hydrogen bonds with water molecules.
Butanol is less soluble because the longer hydrocarbon chain makes the molecule more non-polar.
As chain length increases, the polar –OH group has less influence on overall polarity → decreased solubility.
Example
Why does pentanoic acid have a higher boiling point than propanoic acid?
▶️Answer/Explanation
Both compounds contain the carboxylic acid group (–COOH) capable of hydrogen bonding.
Pentanoic acid has a longer hydrocarbon chain than propanoic acid → higher molar mass.
Stronger London dispersion forces in pentanoic acid → more energy required to boil.
Conclusion: More carbons → stronger intermolecular forces → higher boiling point.
Applications of Homologous Series
Predicting Physical Properties
- Boiling and melting points: These increase gradually with molecular size due to stronger intermolecular forces (e.g., London dispersion forces).
- Solubility: For polar series (like alcohols), solubility in water decreases as the non-polar hydrocarbon chain becomes longer.
- Volatility and density: These also show predictable trends across a homologous series.
- Members of a series share similar chemical reactivity due to having the same functional group.
- For example, all alcohols can undergo oxidation, substitution, and esterification reactions.
Environmental and Industrial Relevance
- Fuels: Alkanes such as methane, propane, and butane are widely used as fuels. They are considered relatively clean compared to coal or oil because they burn more completely, producing mainly $\text{CO}_2$ and $\text{H}_2\text{O}$ with fewer impurities (e.g., sulfur compounds). However, they still contribute to greenhouse gas emissions.
- Pharmaceuticals: Functional groups in homologous series determine biological activity, making organic molecules the basis of many medicines.
- Plastics and polymers: Simple alkenes such as ethene serve as raw materials for plastics and polymers, which are important industrial products.
Implications
- Understanding homologous series helps students master naming, drawing structures, and predicting reactions.
- It forms the backbone for exploring organic mechanisms, reaction pathways, and synthetic strategies.
- In real life, it explains trends in fuel efficiency, solubility of drugs, and the behavior of everyday chemicals.
