Functional groups: S3.2.5 IUPAC nomenclature IB DP Chemistry Study Notes - New Syllabus 2025
Functional groups: Classification of organic compounds – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
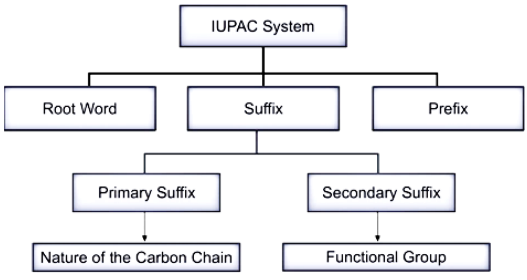
Structure 3.2.5 – IUPAC Nomenclature
Structure 3.2.5 – IUPAC Nomenclature
IUPAC nomenclature refers to the standardized system created by the International Union of Pure and Applied Chemistry to assign unique and descriptive names to chemical compounds. In organic chemistry, this system ensures that the name of any compound corresponds exactly to its molecular structure — no matter how complex.
This systematic naming process allows chemists across the world to communicate molecular structures unambiguously through names alone. It applies to both simple hydrocarbons and complex functionalized organic molecules.
Core Principles of the IUPAC System
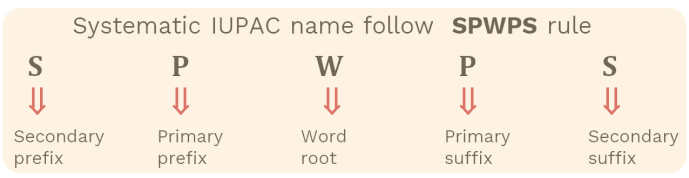
The IUPAC naming method follows a stepwise structure that applies a specific order of rules. A compound’s name is constructed from several parts:
- Parent Hydrocarbon: Based on the longest continuous carbon chain in the molecule.
- Saturation: Indicates whether the carbon chain contains single, double, or triple bonds.
- Functional Groups: Groups of atoms that modify the compound’s chemical properties and determine the suffix or infix of the name.
- Substituents: Side chains or functional groups not part of the main chain, written as prefixes.
- Locants (Numbers): Numbers are used to show the exact position of branches, multiple bonds, or functional groups in the carbon chain.
Names are always written as one word with hyphens and commas used only for spacing locants and separating prefixes.
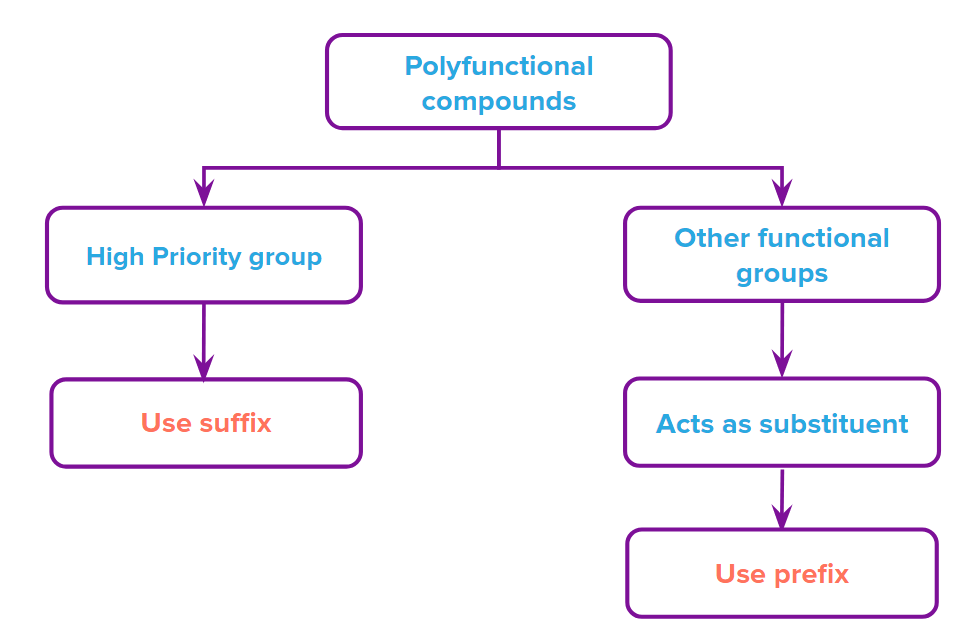
Priority Order of Functional Groups in IUPAC Naming
When more than one functional group is present in a molecule, IUPAC rules require prioritization to determine the main suffix and proper numbering. The functional group with the highest priority gets the suffix; others are named as prefixes.
| Priority Rank | Functional Group | Prefix | Suffix (Main Group) |
|---|---|---|---|
| 1 | Carboxylic acid (–COOH) | carboxy– | –oic acid |
| 2 | Ester(–COO–) | alkoxycarbonyl– | –oate, –carboxylate |
| 3 | Amides(–CONH₂) | amido– | –amide, –carboxyamide |
| 4 | Aldehyde (–CHO) | formyl– | –al |
| 5 | Ketone (>C=O) | oxo– | –one |
| 6 | Alcohol (–OH) | hydroxy– | –ol |
| 7 | Amine (–NH₂) | amino– | –amine |
| 8 | Ether(–O–) | alkoxy– | – |
| 9 | Alkene (C=C) | – | –ene |
| 10 | Alkyne (C≡C) | – | –yne |
| 11 | Halogens (–Cl, –Br, –I) | chloro–, bromo–, iodo– | – |
| 12 | Alkyl groups (–CH₃, –CH₂CH₃) | methyl–, ethyl– | – |
Example: In a molecule containing both –COOH and –OH, the –COOH group gets priority and the compound is named as a carboxylic acid. The –OH group becomes a prefix (“hydroxy–”).
Step-by-Step Rules for Naming Organic Compounds
- Identifying the Parent Chain:
- Select the longest continuous chain of carbon atoms containing the highest priority functional group.
- This chain determines the base name or “parent hydrocarbon” of the compound.
- Numbering the Carbon Chain:
- Assign numbers to carbon atoms in the parent chain to give the principal functional group the lowest possible number.
- If multiple groups are present, priority is given according to IUPAC functional group hierarchy.
- Identifying and Naming Substituents:
- Substituents (side chains, branches, or functional groups not included in the parent chain) are named and positioned as prefixes.
- Examples include alkyl groups (e.g., methyl, ethyl) and halogens (e.g., fluoro, chloro).
- Assigning Multiplicative Prefixes:
- If two or more identical substituents are present, use prefixes such as di–, tri–, tetra–, etc.
- List all positions separated by commas (e.g., 2,3-dimethyl).
- Determining the Suffix:
- The suffix reflects the primary functional group and the degree of saturation of the molecule (e.g., -ane, -ene, -yne, -ol, -al, -one).
- Double/triple bonds and functional groups may also be indicated with locants (e.g., but-2-ene, pent-3-yne).
- Combining the Components:
- The name is assembled in the order: prefix (substituents) + parent hydrocarbon + suffix (functional group).
- Hyphens and numbers are used to clarify positions and prevent ambiguity.
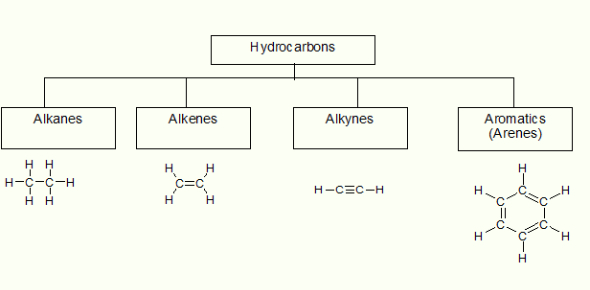
Hydrocarbons: The Basis of Organic Naming
Hydrocarbons are compounds made entirely of carbon and hydrogen. They form the foundation for more complex organic molecules and are classified as follows:
- Alkanes – contain only single bonds (saturated)
- Alkenes – contain at least one double bond (unsaturated)
- Alkynes – contain at least one triple bond (unsaturated)
- Arenes – contain delocalized π-electrons in a benzene ring (aromatic compounds)

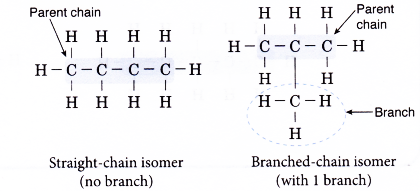
Straight-Chain vs Branched-Chain Isomers
- Many of the compounds have isomers — molecules with the same molecular formula but different structural arrangements.
- Straight-chain isomers have unbranched carbon chains, while branched-chain isomers have side chains (alkyl groups).
- In branched isomers, side chains are treated as substituents and are included as prefixes (e.g., methyl-, ethyl-) with their position indicated by a number.

1. Naming Alkanes (Saturated Hydrocarbons)
- Alkanes are named using the suffix –ane and a stem that reflects the number of carbon atoms in the longest chain.
- The general formula is \( C_nH_{2n+2} \).
| Carbon Count | Formula | Stem | Name |
|---|---|---|---|
| 1 | CH₄ | meth- | methane |
| 2 | C₂H₆ | eth- | ethane |
| 3 | C₃H₈ | prop- | propane |
| 4 | C₄H₁₀ | but- | butane |
| 5 | C₅H₁₂ | pent- | pentane |
| 6 | C₆H₁₄ | hex- | hexane |
Example
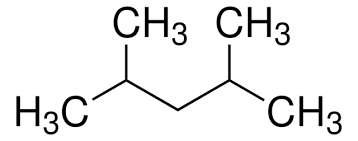
Give the IUPAC name for the compound:
\( \text{CH}_3\text{CH(CH}_3)\text{CH}_2\text{CH(CH}_3)\text{CH}_3 \)
▶️Answer/Explanation
- First, identify the longest continuous chain: 5 carbon atoms → base name is pentane.
- There are two methyl branches: one on carbon 2 and one on carbon 4.
- Number the chain from the end that gives the lowest possible numbers to the branches.
- Since the methyl groups are identical, we use the prefix di- and list their positions.
- IUPAC name: 2,4-dimethylpentane

2. Naming Alkenes (Mono – Unsaturated Hydrocarbons)
- Alkenes contain one or more double bonds and use the suffix –ene.
- The general formula is \( C_nH_{2n} \).
- The location of the double bond is indicated with a number.
| Carbon Count | Formula | IUPAC Name |
|---|---|---|
| 2 | C₂H₄ | ethene |
| 3 | C₃H₆ | propene |
| 4 | C₄H₈ | but-1-ene |
| 5 | C₅H₁₀ | pent-2-ene |
| 6 | C₆H₁₂ | hex-3-ene |
3. Naming Alkynes
- Alkynes contain one or more triple bonds and are named with the suffix –yne.
- The general formula is \( C_nH_{2n-2} \).
- The position of the triple bond must be indicated.
| Carbon Count | Formula | IUPAC Name |
|---|---|---|
| 2 | C₂H₂ | ethyne |
| 3 | C₃H₄ | propyne |
| 4 | C₄H₆ | but-1-yne |
| 5 | C₅H₈ | pent-2-yne |
| 6 | C₆H₁₀ | hex-3-yne |
Example
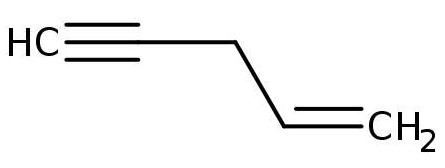
Give the IUPAC name for the compound:
\( \text{CH}_3\text{CH} = \text{CHCH}_2\text{C} \equiv \text{CH} \)
▶️Answer/Explanation
- The molecule contains both a double bond and a triple bond.
- Longest chain: 5 carbon atoms → base name is pent.
- Numbering from the end nearer the double or triple bond gives:
Double bond at position 1, triple bond at position 4. - In IUPAC, the double bond is given priority in numbering when both are present.
- Name: pent-1-en-4-yne

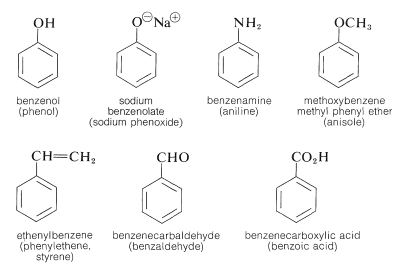
4. Naming Arenes
- Aromatic compounds contain a benzene ring.
- When benzene is the parent structure, its name ends in –benzene (e.g., methylbenzene).
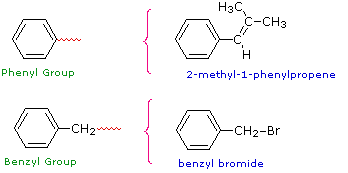
- When benzene is a substituent, it is named as a phenyl group.

- If more than one substituent is present on the ring, the positions are numbered to give the lowest possible values, and groups are listed in alphabetical order

Example
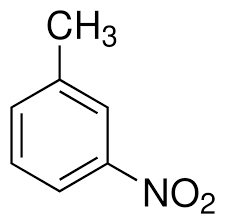
Give the IUPAC name for the compound with the structure:
A benzene ring with a methyl group at position 1 and a nitro group at position 3.
▶️Answer/Explanation
- Parent structure is benzene.
- Methyl group (–CH₃) is the first substituent → base name is methylbenzene (common name: toluene).
- Nitro group (–NO₂) is on carbon 3 relative to the methyl → numbered to give lowest possible set.
- Final IUPAC name: 3-nitrotoluene (or 3-nitromethylbenzene).

Applying IUPAC Nomenclature to Functionalized Compounds
Applying IUPAC Nomenclature to Functionalized Compounds
The naming process still follows the same core rules as described before, but now incorporates functional group suffixes and position numbering. The highest-priority group determines the suffix, while others are used as prefixes if present.
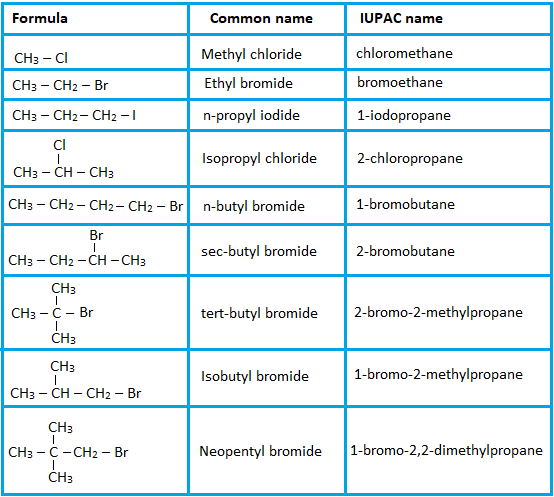
1. Halogenoalkanes (Alkyl Halides)
- Halogenoalkanes are alkanes with halogen atoms (F, Cl, Br, I) replacing one or more hydrogens.
- They are named using the prefix: fluoro–, chloro–, bromo–, or iodo–, followed by the name of the parent alkane.
- Position numbers are assigned to indicate where the halogen is located. The carbon chain is numbered to give the substituent the lowest possible number.

Example
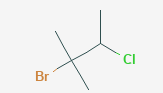
Give the IUPAC name for the compound:
\( \text{CH}_3\text{C}(\text{Br})(\text{CH}_3)\text{CH}(\text{Cl})\text{CH}_3 \)
▶️Answer/Explanation
- Start by identifying the longest carbon chain: There are 4 carbon atoms → base name is butane.
- There is a methyl branch on carbon 2, a bromine on carbon 2, and a chlorine on carbon 3.
- Number the chain from the end closest to the substituents → from the left side, to give lowest possible locants.
- List substituents in alphabetical order: bromo, chloro, methyl.
- IUPAC name: 2-bromo-3-chloro-2-methylbutane

2. Alcohols
- Alcohols contain the hydroxyl group (–OH).
- They are named using the suffix –ol.
- The –OH group is given the lowest possible position number on the carbon chain.
- For molecules with more than one hydroxyl groups, use the suffix –diol, triol and so on.

Example
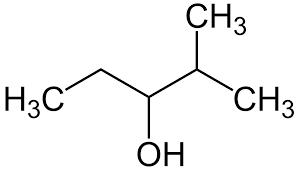
Give the IUPAC name for the compound:
\( \text{CH}_3\text{CH}(\text{CH}_3)\text{CH}(\text{OH})\text{CH}_2\text{CH}_3 \)
▶️Answer/Explanation
- Longest carbon chain: 5 carbon atoms → base name is pentane.
- There is a hydroxyl (–OH) group on carbon 3 → suffix becomes –3-ol.
- A methyl group is attached to carbon 2 → prefix 2-methyl.
- Numbering starts from the end closest to the –OH group, since it has functional group priority.
- IUPAC name: 2-methylpentan-3-ol

3. Aldehydes
- Aldehydes contain a terminal carbonyl group (–CHO).
- They are named using the suffix –al.
- Since the carbonyl group is always on carbon-1, no locant is needed.

Example
Give the IUPAC name for the compound:
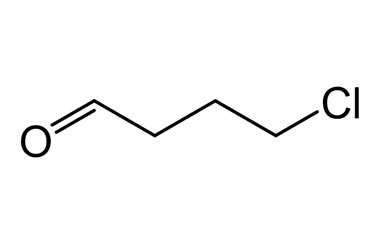
\( \text{ClCH}_2\text{CH}_2\text{CH}_2\text{CHO} \)
▶️Answer/Explanation
- The compound contains an aldehyde group (–CHO), which is always located at carbon 1.
- The longest chain includes 4 carbon atoms including the carbon of the aldehyde group → base name is butanal.
- There is a chloro substituent on carbon 4 (when numbering from the aldehyde carbon).
- IUPAC name: 4-chlorobutanal

4. Ketones
- Ketones have a carbonyl group (C=O) located within the carbon chain.
- They are named using the suffix –one.
- The position of the carbonyl group is indicated by a number unless the structure makes the position obvious.

Example
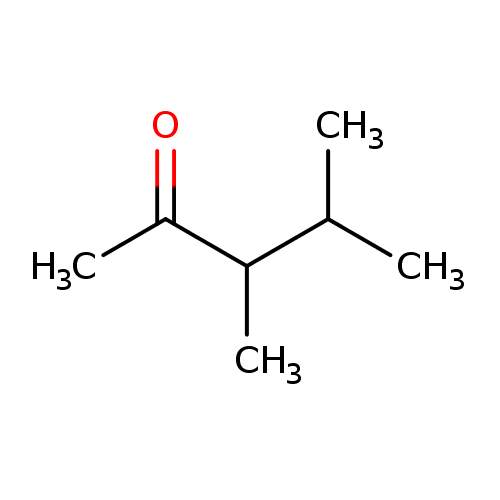
Give the IUPAC name for the compound:
\( \text{CH}_3\text{COCH}(\text{CH}_3)\text{CH}(\text{CH}_3)\text{CH}_3 \)
▶️Answer/Explanation
- Step 1: Identify the longest chain that includes the ketone carbon. The longest chain is 5 carbons → base name is pentanone.
- Step 2: The carbonyl (C=O) is on carbon 2 → suffix becomes pentan-2-one.
- Step 3: There are two methyl groups as side chains:
- One on carbon 3
- One on carbon 4
- Step 4: Numbering starts from the end nearest the carbonyl group to give it the lowest possible number (priority).
- Step 5: List substituents alphabetically with their positions. Two methyl groups → 3,4-dimethyl.
- IUPAC name: 3,4-dimethylpentan-2-one

5. Carboxylic Acids
- Carboxylic acids contain the carboxyl group (–COOH) .
- They are named with the suffix –oic acid.
- The –COOH group is always carbon-1, so it does not require a locant.

Example
Give the IUPAC name for the compound:
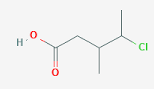
\( \text{ClCH}_2\text{CH}(\text{CH}_3)\text{CH}(\text{CO}_2\text{H})\text{CH}_2\text{CH}_3 \)
▶️Answer/Explanation
- Step 1: Identify the longest carbon chain that contains the carboxylic acid group (–COOH). The chain has 5 carbon atoms including the –COOH → base name is pentanoic acid.
- Step 2: Number the chain starting from the carboxylic acid carbon (carbon 1) — since the –COOH group always takes priority.
- Step 3: Identify substituents:
- A methyl group on carbon 3 → 3-methyl
- A chlorine (–Cl) atom on carbon 4 → 4-chloro
- Step 4: List substituents alphabetically and specify their positions.
- Final IUPAC name: 4-chloro-3-methylpentanoic acid

6. Ester
- Esters are compounds that contain the functional group –COO– linking a carbon chain to an alkyl or aryl group. They are formed from a carboxylic acid and an alcohol in a condensation reaction.
- The name of an ester has two parts:
- The alkyl group from the alcohol comes first.
- The acid part is named using the suffix –oate, based on the parent acid.
Example
Give the IUPAC name for the compound:
\( \text{CH}_3\text{CH}_2\text{COOCH}(\text{CH}_3)_2 \)
▶️Answer/Explanation
- The molecule is an ester, with the structure: acid part (left of COO) and alcohol part (right of COO).
- Acid part: \( \text{CH}_3\text{CH}_2\text{CO–} \) → 3 carbon atoms → base name: propanoate.
- Alcohol part: \( \text{CH(CH}_3)_2 \) → isopropyl group.
- IUPAC name: isopropyl propanoate

7. Amine
- Amines are organic compounds that contain a nitrogen atom bonded to one or more alkyl groups(–NH₂). There are primary (1°), secondary (2°), and tertiary (3°) amines depending on how many carbon atoms are attached to the nitrogen.
- For naming:
- Use the alkyl group name followed by the suffix –amine.
- If there are two identical groups, use di-, e.g., dimethylamine.
Example
Give the IUPAC name for the compound:
\( \text{CH}_3\text{CH}_2\text{NHCH}_2\text{CH}_2\text{CH}_3 \)
▶️Answer/Explanation
- This is a secondary amine, with two different alkyl groups attached to the nitrogen atom.
- One alkyl group is ethyl (CH₃CH₂–), and the other is propyl (CH₃CH₂CH₂–).
- In IUPAC naming, we use the longer carbon chain as the parent name: propan-1-amine.
- The shorter alkyl group bonded to nitrogen is named as a substituent with an “N-” prefix.
- IUPAC name: N-ethylpropan-1-amine

8. Amide
- Amides are compounds that contain the –CONH₂ group (a carbonyl group bonded to a nitrogen atom). They are derived from carboxylic acids where the –OH is replaced by –NH₂.
- Naming amides involves:
- Using the stem of the parent carboxylic acid.
- Replacing –oic acid with –amide.
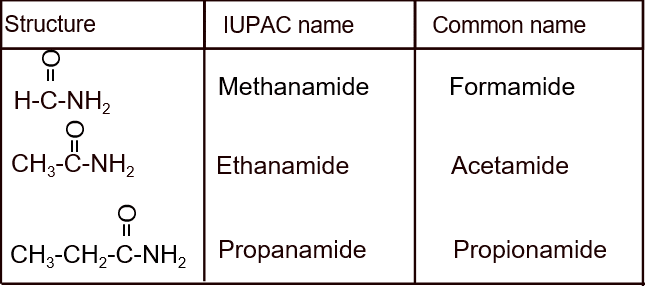
Example
Give the IUPAC name for the compound:
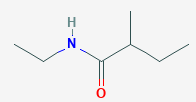
\( \text{CH}_3\text{CH(CH}_3)\text{CH}_2\text{CONHCH}_2\text{CH}_3 \)
▶️Answer/Explanation
- The main carbon chain is 4 carbons long with an amide group (–CONH–) → base: butanamide.
- There is a methyl group on carbon 2 → 2-methylbutanamide.
- The nitrogen is substituted with an ethyl group → N-ethyl.
- Final IUPAC name: N-ethyl-2-methylbutanamide

9. Ether
- Ethers are compounds where an oxygen atom connects two alkyl or aryl groups (R–O–R′). They do not have a functional group suffix in basic IUPAC naming and are often named as alkoxyalkanes.
- Naming rule:
- The shorter alkyl group with oxygen is treated as an alkoxy substituent (like methoxy, ethoxy).
- The longer alkyl chain is used as the base name.
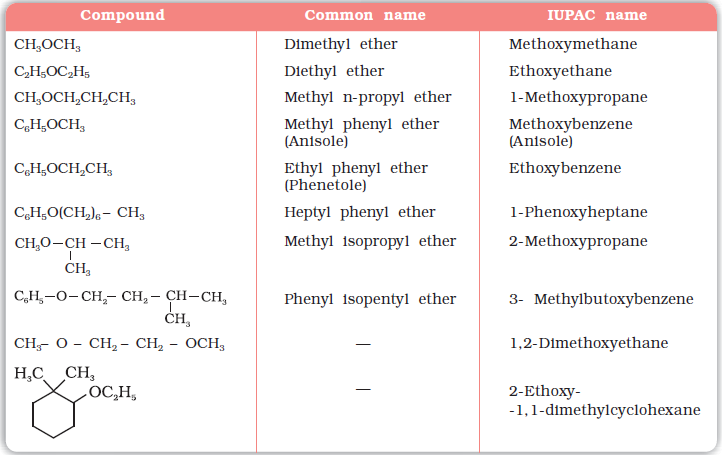
Example
Give the IUPAC name for the compound:
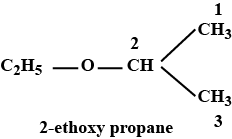
\( \text{CH}_3\text{CH}_2\text{OCH}(\text{CH}_3)_2 \)
▶️Answer/Explanation
- The compound is an ether (R–O–R’). It has two alkyl groups joined by an oxygen atom.
- Identify the shorter alkyl chain as the substituent. Here:
- Ethyl group (CH₃CH₂–)
- Isopropyl group (CH(CH₃)₂)
- In IUPAC naming, ethers are named as alkoxyalkanes.
- The ethoxy (–OCH₂CH₃) group is treated as a substituent on the isopropane chain.
- Longest carbon chain: isopropane (3 carbon atoms) → base: propane.
- Number the chain from the end nearest the ethoxy group. The –OCH₂CH₃ is on carbon 2.
- IUPAC name: 2-ethoxypropane