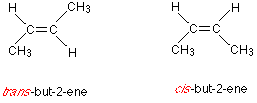Functional groups: S3.2.7 Stereoisomers IB DP Chemistry Study Notes - New Syllabus 2025
Functional groups: Classification of organic compounds – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Structure 3.2.7 – Stereoisomerism
Structure 3.2.7 – Stereoisomerism
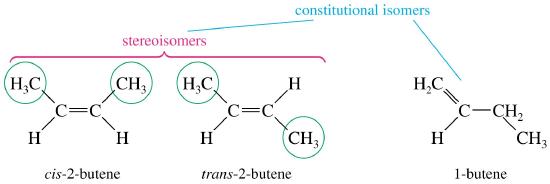
- Stereoisomers are molecules that have the same molecular formula and the same structural formula (i.e., same atom identities, same bonds, and same bonding sequence), but they differ in the spatial arrangement of atoms in three dimensions.
- This means that while all atoms are connected in the same order, their orientation in space is different, leading to different physical and chemical behaviors in some contexts (e.g., optical activity).
- Stereoisomerism arises due to the restricted rotation around double bonds or the presence of chiral centers.
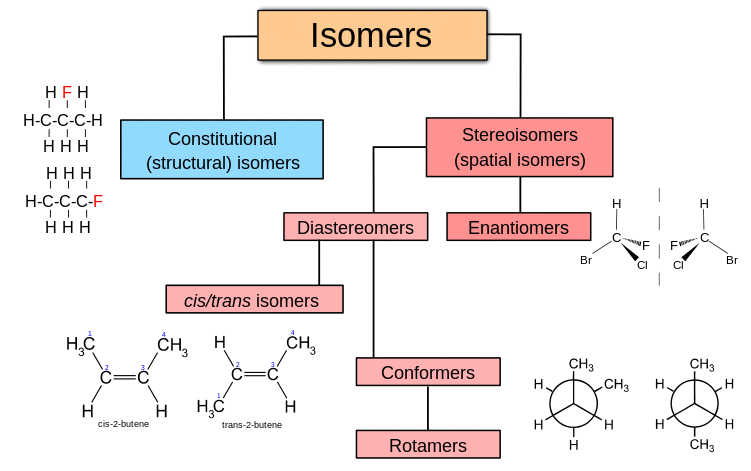
Stereoisomers are of particular importance in biological systems and drug chemistry because their three-dimensional structure can affect how they interact with enzymes and receptors.
Example
How are stereoisomers different from structural isomers?
▶️Answer/Explanation
- Structural isomers: Same molecular formula but different connectivity of atoms.
- Stereoisomers: Same connectivity but different 3D spatial arrangements.
- For example, but-2-ene has two stereoisomers (cis and trans) due to restricted rotation about the C=C bond.

Conformational Isomers
Conformational Isomers
Conformational isomers (also called conformers) are a type of stereoisomer that differ by the rotation around a single sigma (σ) bond. Unlike structural or geometric isomers, conformers interconvert easily at room temperature and do not involve breaking bonds.
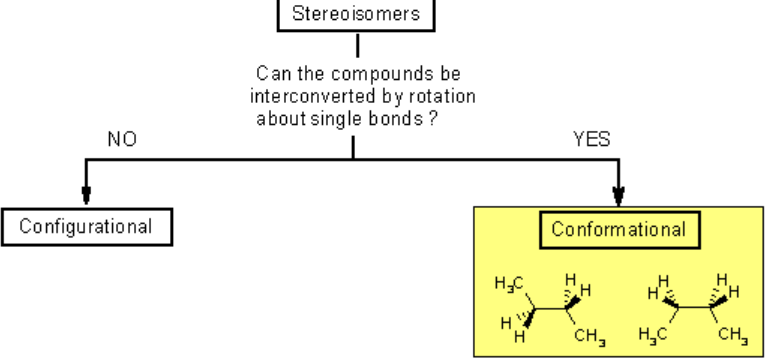
Key Characteristics
- They have the same molecular and structural formula.
- They result from rotation about single bonds (not double bonds).
- They are usually not isolable because the energy barrier between them is small.
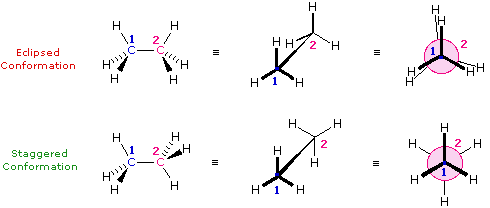
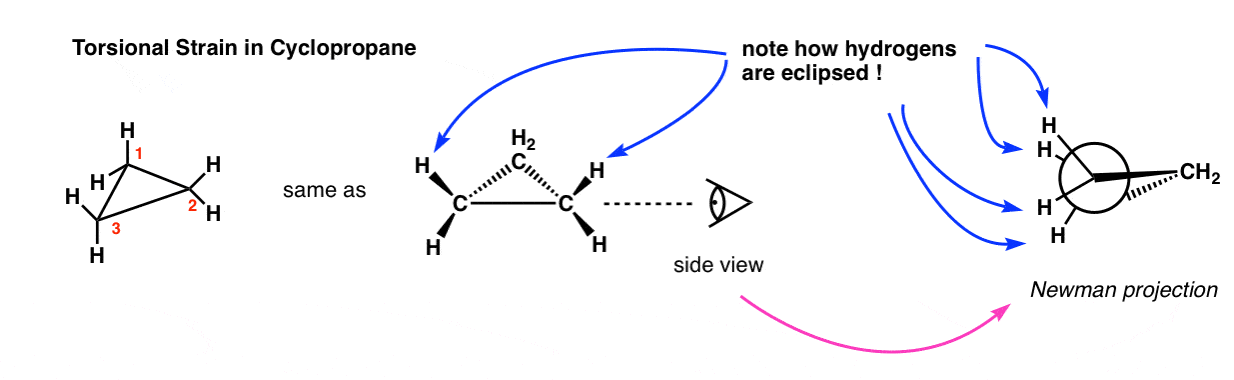
Conformations of Ethane
The simplest example of conformational isomerism occurs in ethane \( (\text{CH}_3\text{CH}_3) \). As one methyl group rotates around the carbon–carbon single bond, different conformations are formed:
- Staggered conformation: The hydrogen atoms on adjacent carbon atoms are as far apart as possible — this is the most stable due to minimal electron repulsion.
- Eclipsed conformation: The hydrogen atoms on adjacent carbons align with each other — this is least stable due to torsional strain.

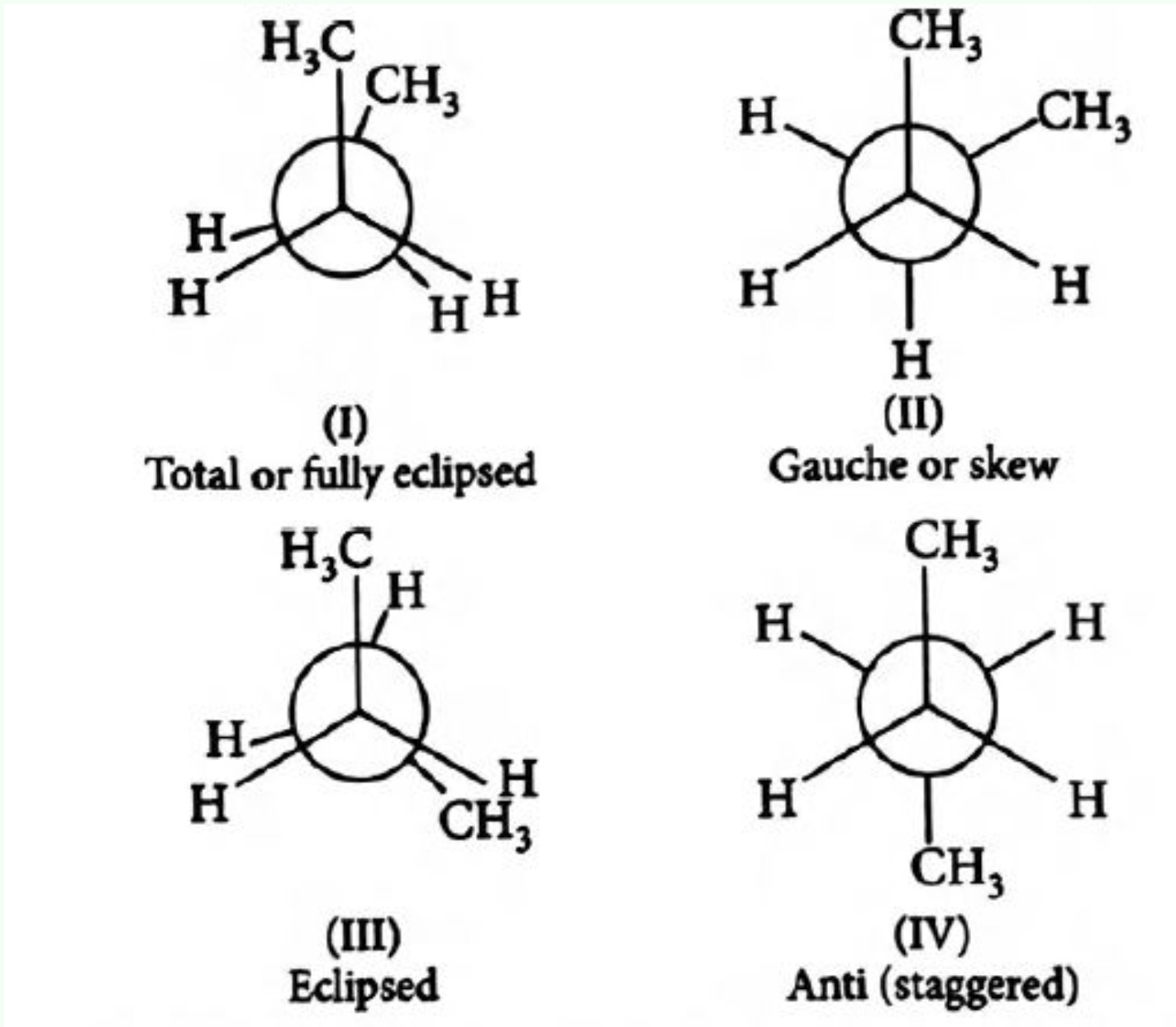
Conformations of Butane
In butane \( (\text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_3) \), rotation around the central C–C bond leads to multiple conformers:
- Anti conformation: Two methyl groups are 180° apart — most stable.
- Gauche conformation: Two methyl groups are 60° apart — less stable.
- Eclipsed conformation: Methyl groups are aligned — least stable.
-

Energy Profile
The energy of conformational isomers can be plotted as a function of bond rotation. Staggered forms are at energy minima, while eclipsed forms represent energy maxima due to increased electron repulsion.
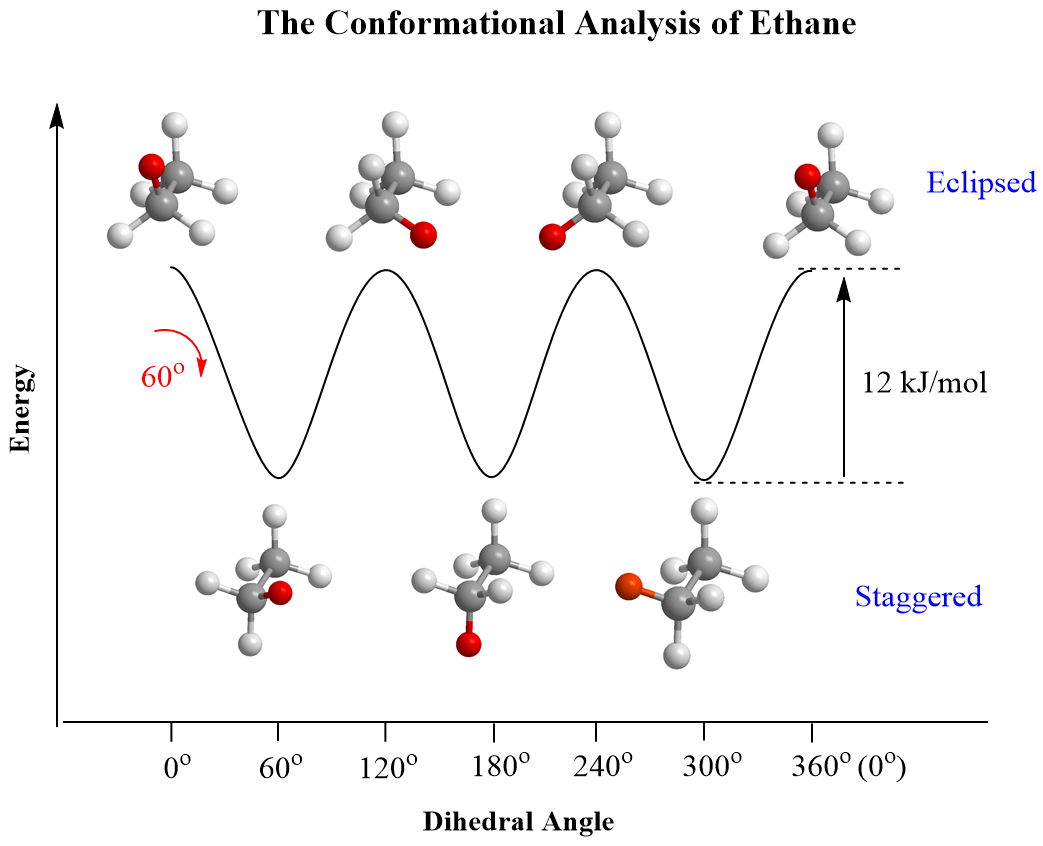
Example
Describe and compare the staggered and eclipsed conformations of ethane. Which is more stable and why?
▶️Answer/Explanation
- In ethane \( (\text{CH}_3\text{CH}_3) \), rotation around the C–C sigma bond produces conformers.
- Staggered conformation: The hydrogen atoms are 60° apart. This minimizes electron repulsion between bonding pairs of electrons. It is the most stable form.
- Eclipsed conformation: The hydrogen atoms on adjacent carbons overlap in the Newman projection. This causes torsional strain due to repulsion between electron clouds in the C–H bonds. It is the least stable.
- The molecule naturally spends more time in the staggered form due to lower potential energy.
Conformational Isomerism in Cyclic Structures
Conformational isomerism in cyclic compounds arises from the flexibility of ring structures, which try to minimize angle and torsional strain by adopting different spatial arrangements (conformations) without breaking bonds.
Why Rings Undergo Conformational Isomerism
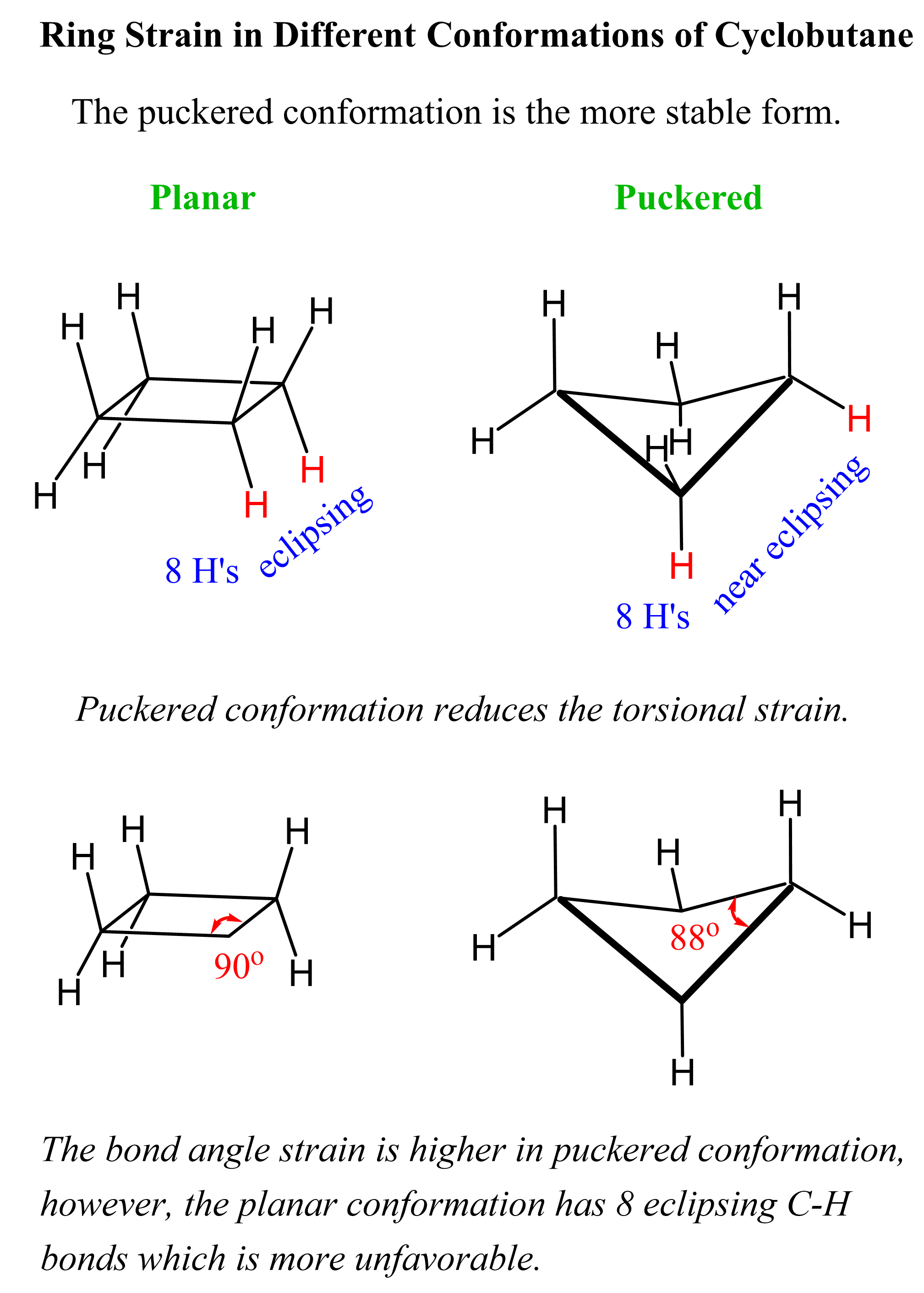
Cyclic compounds, especially those with more than three carbon atoms, cannot remain flat without introducing strain. As a result, they adopt non-planar conformations to reduce:
- Angle strain (deviation from ideal tetrahedral angle of 109.5°)
- Torsional strain (eclipsing interactions of bonds)
Common Cyclic Conformers
1. Cyclopropane and Cyclobutane
- These small rings are rigid due to geometric constraints.
- They are planar and suffer from severe angle strain (especially cyclopropane with 60° angles).


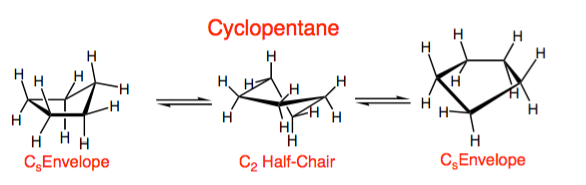
2. Cyclopentane
- Cyclopentane adopts a puckered envelope conformation to reduce torsional strain.
- One carbon is slightly out of the plane of the other four.

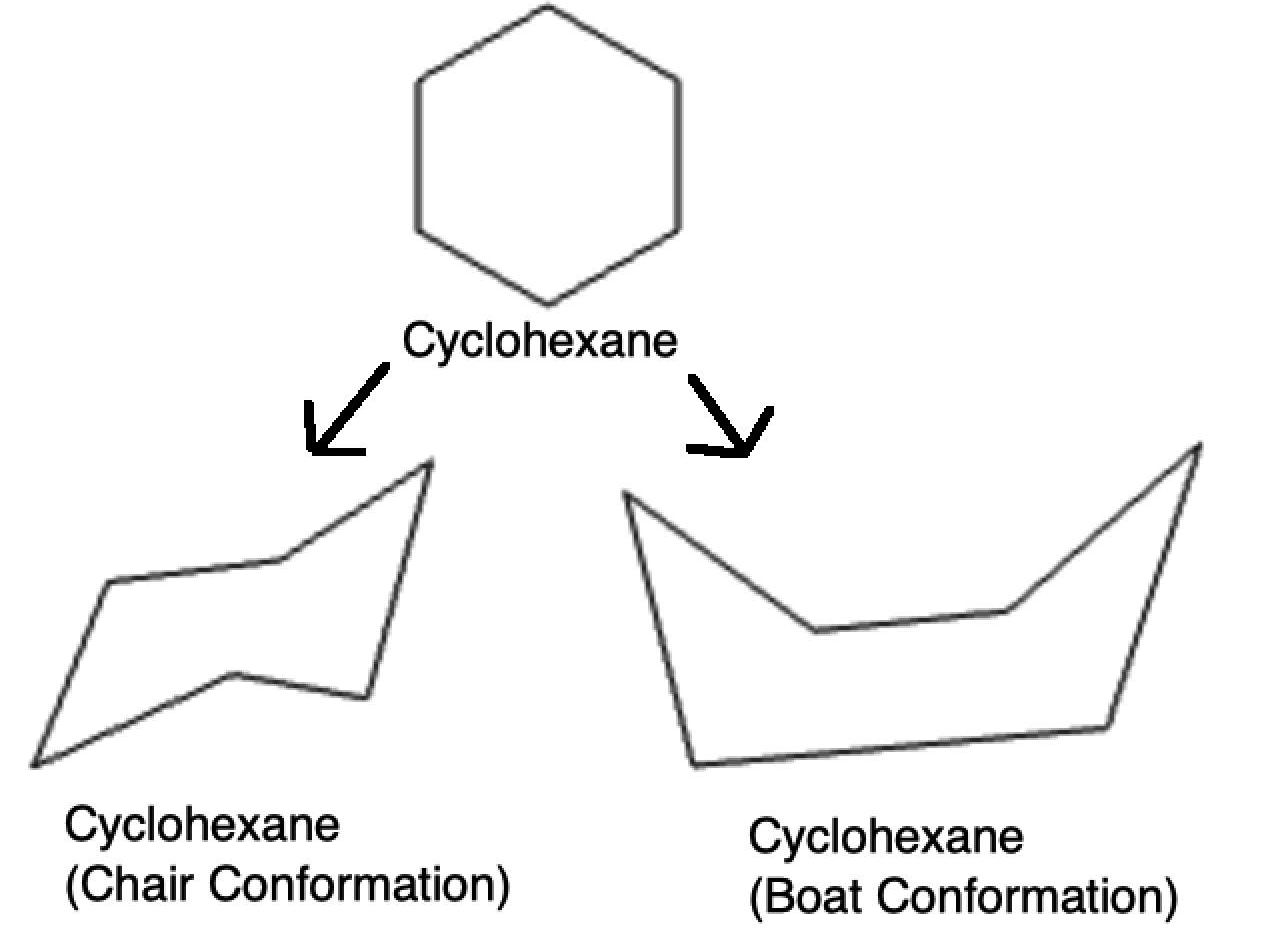
3. Cyclohexane
Cyclohexane is the most important example. It can adopt multiple conformations, the most stable being:
- Chair conformation: Minimizes both angle and torsional strain. All C–C–C bond angles are ~109.5°.
- Boat conformation: Less stable due to eclipsed interactions and steric strain between flagpole hydrogens.

Axial and Equatorial Positions
In the chair form of cyclohexane, each carbon has two substituents:
- Axial: Perpendicular to the ring (up or down)
- Equatorial: Parallel to the ring’s plane, pointing outward
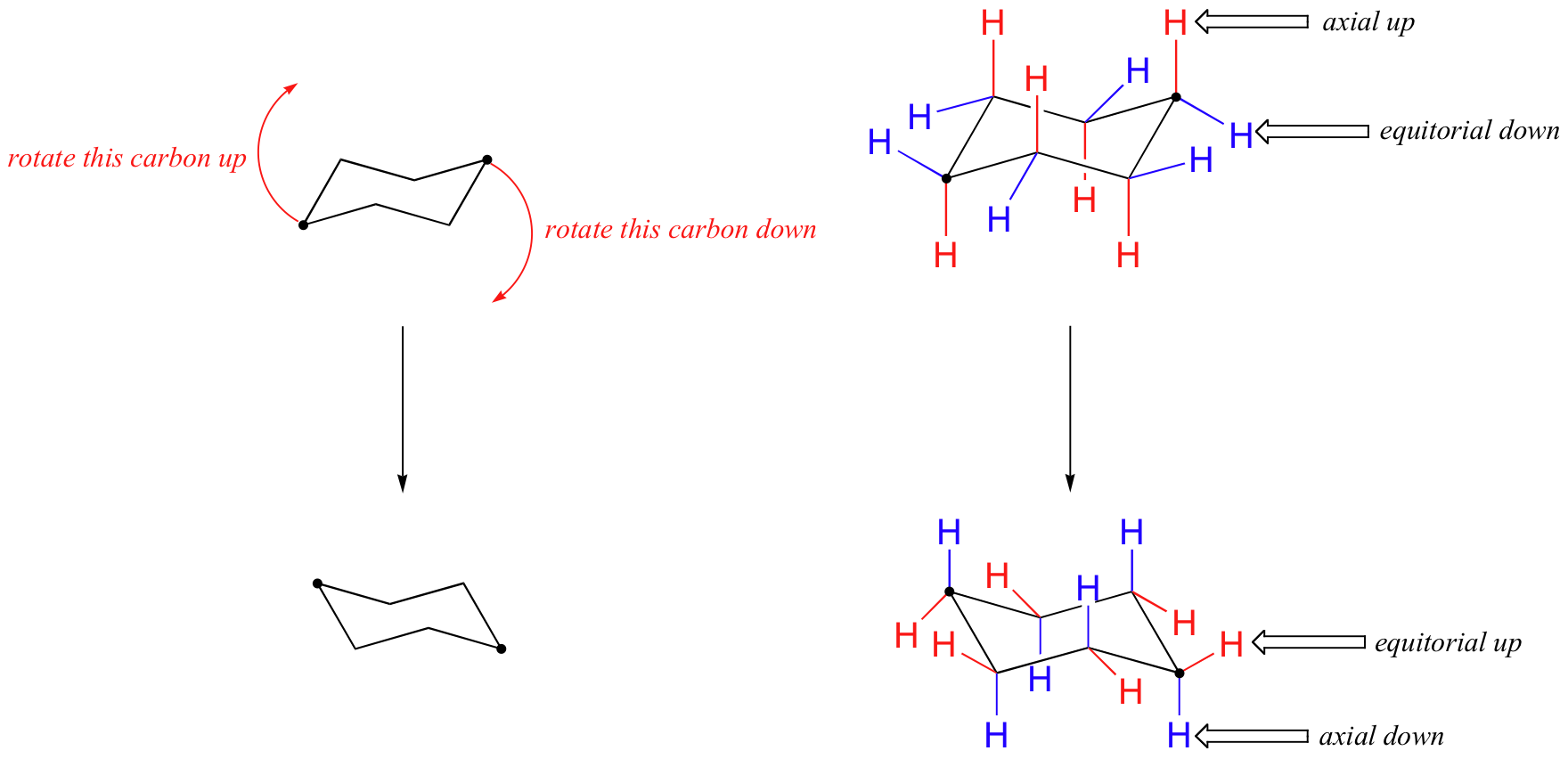
Substituents prefer the equatorial position to reduce steric hindrance, especially in monosubstituted cyclohexanes.
Ring Flip
Cyclohexane undergoes a dynamic process called a ring flip, where axial and equatorial positions switch. This interconversion affects the stability of different conformers, especially when bulky groups are present.
Example
Explain why the chair conformation of methylcyclohexane is more stable when the methyl group is in the equatorial position rather than axial.
▶️Answer/Explanation
- In the chair conformation of cyclohexane, substituents can occupy either axial or equatorial positions.
- When the methyl group is in the axial position, it experiences 1,3-diaxial interactions (steric hindrance) with other axial hydrogens on the same side of the ring.
- These repulsions increase the potential energy of the molecule, making it less stable.
- In contrast, placing the methyl group equatorially allows it to avoid such interactions, minimizing steric strain.
- Thus, the conformation with the methyl in the equatorial position is more stable.
Cis/Trans Isomerism
Cis/Trans Isomerism
Cis–trans isomerism (a type of geometric isomerism) occurs when molecules have the same structural formula but differ in the spatial arrangement of atoms or groups around a restricted rotation site, such as a double bond or ring.

Why Cis/Trans Isomerism Occurs
- In alkenes: due to the lack of rotation around the C=C double bond.
- In cyclic compounds: due to restricted ring structures, which prevent free rotation between substituents.
Conditions for Cis/Trans Isomerism
To show cis–trans isomerism, a molecule must have:
- A carbon–carbon double bond (or a ring system).
- Each carbon (in the double bond or ring) must be bonded to two different groups.
Cis vs. Trans
- Cis isomer: Identical or similar groups are on the same side of the double bond or ring.
- Trans isomer: Identical or similar groups are on the opposite sides.
Importance
Cis and trans isomers often have different physical properties such as boiling point, solubility, and dipole moment, and may react differently in chemical reactions.
Example
Does but-2-ene show cis–trans isomerism? If so, draw and describe both isomers.
▶️Answer/Explanation
- Structure: \( \text{CH}_3\text{CH}=\text{CH}\text{CH}_3 \)
- Each double-bonded carbon is bonded to a hydrogen and a methyl group (–CH₃).
- This allows for two possible arrangements:
- Cis-but-2-ene: both –CH₃ groups are on the same side.
- Trans-but-2-ene: –CH₃ groups are on opposite sides.
- Therefore, but-2-ene exhibits cis–trans isomerism.

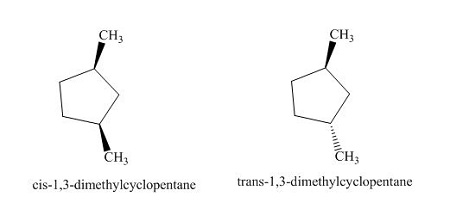
Cis/Trans Isomerism in Cyclic Compounds
Cis/trans isomerism in cyclic compounds arises due to the restricted rotation around the single bonds in the ring structure. Unlike open-chain alkanes where free rotation is possible, the atoms in a ring are held in fixed positions relative to one another, which makes geometric isomerism possible even in saturated rings.
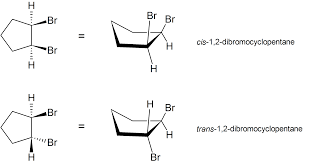
Key Features
- Cis/trans isomerism occurs when two identical or similar groups are attached to non-adjacent carbon atoms in a ring.
- Because the ring prevents free rotation, the relative positions of these groups can be either:
- Cis: The two groups are on the same side of the ring plane (both “up” or both “down”).
- Trans: The two groups are on opposite sides of the ring plane (one “up”, one “down”).
Requirements for Cis/Trans Isomerism in Rings
- The ring must contain at least four carbon atoms (cyclopropane and cyclobutane rarely show distinct isomers due to strain and symmetry).
- Each of the ring carbons bearing substituents must be bonded to two different groups.
- Cis and trans isomers are distinct molecules and often have different physical properties such as melting point, boiling point, and polarity.
Stability and Properties
- Trans isomers are often more stable than cis isomers due to reduced steric hindrance (groups are further apart).
- Cis isomers may exhibit higher polarity when both substituents are on the same side, which affects solubility and intermolecular interactions.
Cis/trans isomerism in cyclic compounds is an important feature in organic and biological molecules, as the spatial orientation of groups can influence chemical reactivity and biological activity.
Example
Does 1,2-dimethylcyclopentane show cis–trans isomerism ? Explain with both isomers.
▶️Answer/Explanation
- Yes. In cyclic compounds, rotation is restricted because of the ring structure.
- In 1,2-dimethylcyclopentane:
- Cis-isomer: both methyl groups are on the same side of the ring (e.g., both “up”).
- Trans-isomer: the methyl groups are on opposite sides of the ring (one “up”, one “down”).
- These are distinct isomers with different 3D shapes and physical properties.

Enantiomers
Enantiomers
Enantiomers are a type of stereoisomer. They have the same molecular and structural formula, but differ in the three-dimensional arrangement of atoms around a chiral center. Enantiomers are non-superimposable mirror images of each other.
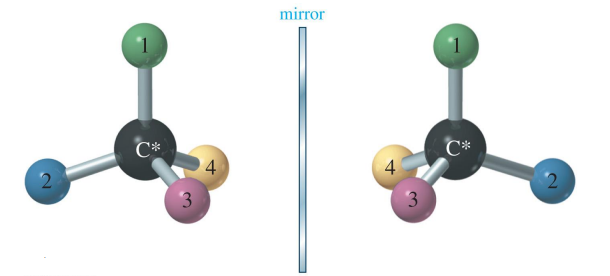
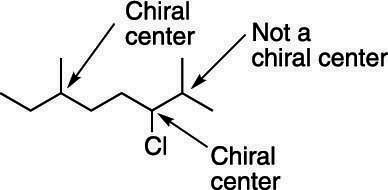
Chirality and Chiral Carbons
- A molecule is said to be chiral if it cannot be superimposed on its mirror image — much like your left and right hands.
- A chiral center (or asymmetric carbon) is a carbon atom bonded to four different groups.
- The presence of a chiral center gives rise to two enantiomers.

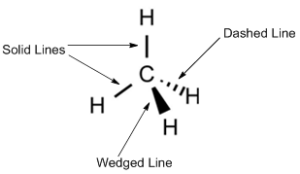
Wedge-Dash Representation
To show the three-dimensional arrangement of atoms around a chiral carbon, chemists use wedge-dash diagrams:
- Solid wedge (▲): group coming out of the plane (toward the viewer)
- Dashed wedge (▼): group going behind the plane (away from the viewer
- Straight lines: groups lying in the plane of the page

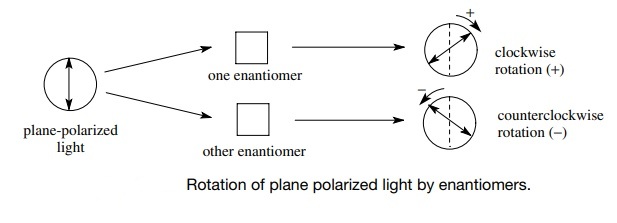
Optical Activity
Enantiomers are optically active — they rotate the plane of plane-polarized light in opposite directions:
- (+)-enantiomer (dextrorotatory): rotates light to the right (clockwise)
- (–)-enantiomer (levorotatory): rotates light to the left (anticlockwise)

However, this optical property cannot be predicted from structure alone; it must be determined experimentally.
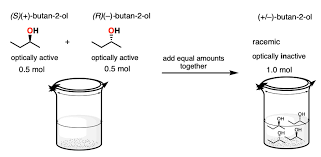
Racemic Mixture
A racemic mixture (or racemate) is an equimolar mixture of two enantiomers — that is, two stereoisomers that are non-superimposable mirror images of each other. Because these enantiomers rotate plane-polarized light in equal and opposite directions, a racemic mixture appears optically inactive.
Key Features
- Contains a 1:1 ratio of the (+) and (–) enantiomers.
- The optical rotations of the individual enantiomers cancel each other out.
- Although enantiomers have identical physical properties, their biological activity may differ significantly. Therefore, a racemic mixture may not behave the same way as a pure enantiomer.
Optical Inactivity
A pure enantiomer will rotate plane-polarized light either clockwise or counterclockwise. However, in a racemic mixture:
- The rotation caused by the dextrorotatory isomer (e.g. (+)) is cancelled by the levorotatory isomer (e.g. (–)).
- The net optical rotation is zero.

Formation of Racemic Mixtures
Racemic mixtures often form in chemical reactions that create a new chiral center unless a chiral reagent or catalyst is used to direct the formation of one enantiomer preferentially.
Separation of Enantiomers (Resolution)
Separating enantiomers in a racemic mixture is called resolution. This typically requires a chiral agent or special technique (e.g., using enzymes or chiral chromatography).
Chemical and Biological Properties
- Enantiomers have identical physical properties (melting point, boiling point, density) and identical chemical properties in non-chiral environments.
- However, they often behave differently in chiral environments, such as:
- Biological systems (e.g., drug receptors)
- Chiral catalysts
Note: IB students are not required to assign R/S configuration, but should understand the concepts of chirality, enantiomers, optical activity, and wedge-dash representations.
Example
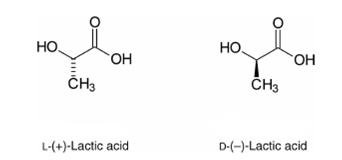
Identify the chiral center in lactic acid and draw the two enantiomers using wedge-dash notation. State whether the molecule is optically active.
▶️Answer/Explanation
- Lactic acid: \( \text{CH}_3\text{CH(OH)COOH} \)
- The central carbon (attached to –CH₃, –OH, –COOH, and –H) is bonded to four different groups — this is the chiral center.
- Two enantiomers are possible, which are non-superimposable mirror images of each other.
- Since it has a chiral center and is not a racemic mixture, lactic acid is optically active.

Example
A chemist synthesizes lactic acid in the lab and obtains a 50:50 mixture of both optical isomers. What is this mixture called, and what is its optical activity?
▶️Answer/Explanation
- This is a racemic mixture because it contains equal amounts of both enantiomers of lactic acid.
- Since each enantiomer rotates plane-polarized light in opposite directions, the mixture is optically inactive.
- This occurs because the optical rotations cancel out.