IB DP Chemistry - R3.3.3 Substitution reactions with alkanes - Study Notes - New Syllabus - 2026, 2027 & 2028
IB DP Chemistry – R3.3.3 Substitution reactions with alkanes – Study Notes – New Syllabus
IITian Academy excellent Introduction to the Proton transfer reactions – Study Notes and effective strategies will help you prepare for your IB DP Chemistry exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 3.3.3 — Substitution Reactions Between Alkanes and Halogens: Propagation and Termination Steps
Reactivity 3.3.3 — Substitution Reactions Between Alkanes and Halogens: Propagation and Termination Steps
Alkanes are generally unreactive because:
- They are non-polar molecules with low reactivity toward electrophiles and nucleophiles.
- They have strong covalent bonds: \( \text{C–C} \approx 348\, \text{kJ/mol} \), \( \text{C–H} \approx 412\, \text{kJ/mol} \).
However, alkanes undergo free radical substitution reactions with halogens in the presence of ultraviolet (UV) light or heat. These reactions follow a radical chain mechanism with three key stages: initiation, propagation, and termination.
Stepwise Mechanism of Free Radical Substitution:
1. Initiation (Already covered in 3.3.2)
Radical generation through homolytic fission of halogen molecules under UV light:
\( \text{Cl}_2 \xrightarrow{\text{UV}} \cdot\text{Cl} + \cdot\text{Cl} \)
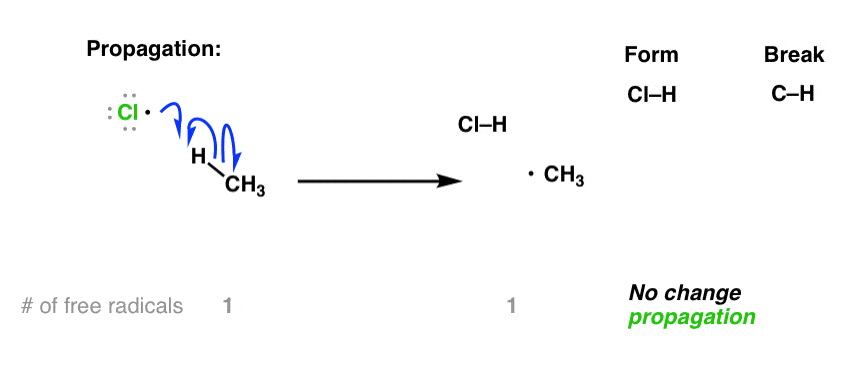
2. Propagation Steps
The chlorine radical reacts with methane (or another alkane), abstracting a hydrogen atom and forming a methyl radical:
\( \cdot\text{Cl} + \text{CH}_4 \rightarrow \text{HCl} + \cdot\text{CH}_3 \)
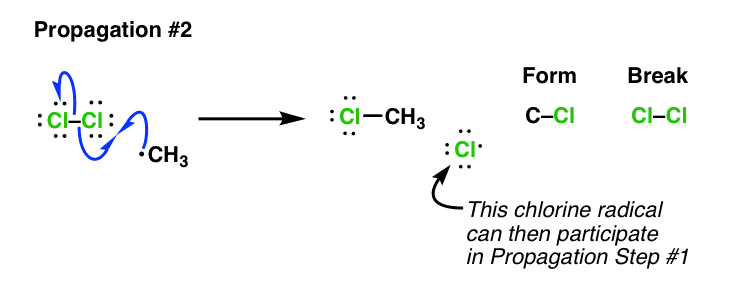
Then, the methyl radical reacts with another chlorine molecule to form chloromethane and regenerate the chlorine radical:
\( \cdot\text{CH}_3 + \text{Cl}_2 \rightarrow \text{CH}_3\text{Cl} + \cdot\text{Cl} \)
These two steps repeat in a chain reaction, continuing until radicals are removed.
3. Termination Steps
Termination occurs when two radicals combine, removing them from the reaction mixture and ending the chain.
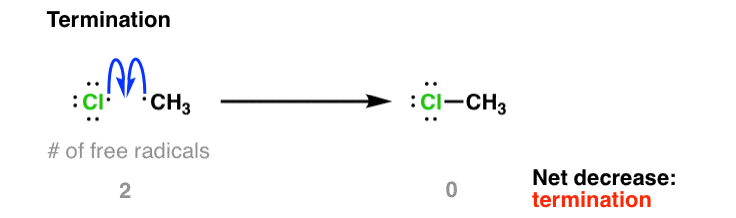
Common termination reactions include:
- \( \cdot\text{Cl} + \cdot\text{Cl} \rightarrow \text{Cl}_2 \)
- \( \cdot\text{CH}_3 + \cdot\text{CH}_3 \rightarrow \text{C}_2\text{H}_6 \)
- \( \cdot\text{Cl} + \cdot\text{CH}_3 \rightarrow \text{CH}_3\text{Cl} \)
Mixture of Products:
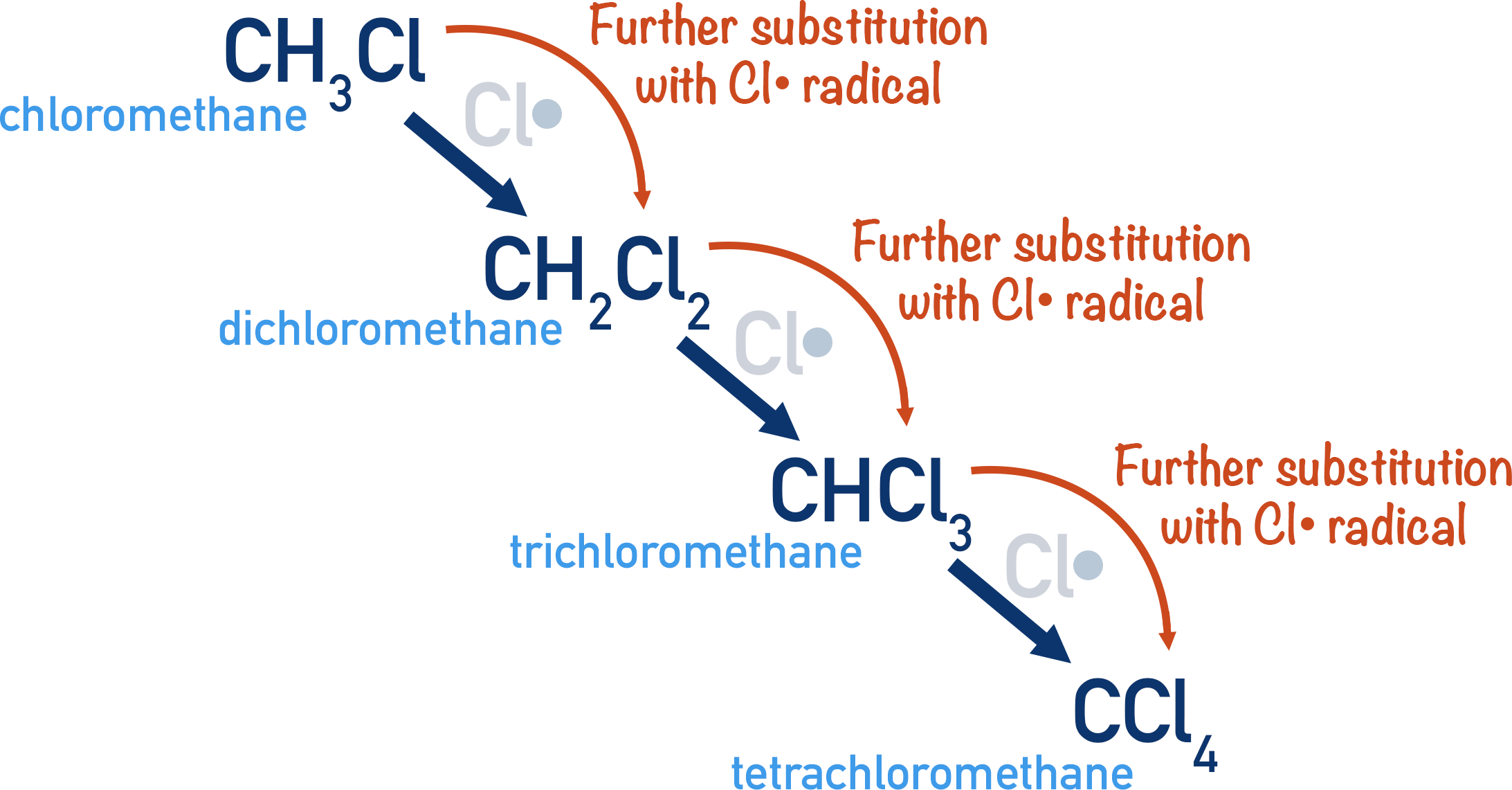
Due to continued substitution, a mixture of products can form, especially with excess halogen:
- Monosubstitution: \( \text{CH}_3\text{Cl} \)
- Disubstitution: \( \text{CH}_2\text{Cl}_2 \)
- Trisubstitution: \( \text{CHCl}_3 \)
- Tetrasubstitution: \( \text{CCl}_4 \)
Stability of Alkanes and Why UV Light Is Required:
- The high bond enthalpies of the C–H and C–C bonds make them resistant to spontaneous breaking.
- Alkanes are non-polar and lack functional groups, further contributing to their low reactivity.
- UV light is needed to initiate the reaction by supplying energy to break the halogen bond and generate radicals.
Example:
Describe the Chlorination of Methane
UV light initiates the substitution of hydrogen atoms in methane by chlorine.
▶️Answer/Explanation
Initiation:
\( \text{Cl}_2 \xrightarrow{hv} 2\cdot \text{Cl} \)
Propagation:
\( \cdot \text{Cl} + \text{CH}_4 \rightarrow \cdot \text{CH}_3 + \text{HCl} \)
\( \cdot \text{CH}_3 + \text{Cl}_2 \rightarrow \text{CH}_3\text{Cl} + \cdot \text{Cl} \)
Termination (examples):
\( \cdot \text{CH}_3 + \cdot \text{CH}_3 \rightarrow \text{C}_2\text{H}_6 \)
\( \cdot \text{Cl} + \cdot \text{Cl} \rightarrow \text{Cl}_2 \)
Note: Excess chlorine and prolonged UV exposure can lead to multiple substitution products: \( \text{CH}_2\text{Cl}_2 \), \( \text{CHCl}_3 \), and \( \text{CCl}_4 \).
Example:
Describe the Bromination of Ethane.
Free radical bromination of ethane shows a similar substitution process with a different alkane and halogen.
▶️Answer/Explanation
Initiation:
\( \text{Br}_2 \xrightarrow{hv} 2\cdot \text{Br} \)
Propagation:
\( \cdot \text{Br} + \text{C}_2\text{H}_6 \rightarrow \cdot \text{C}_2\text{H}_5 + \text{HBr} \)
\( \cdot \text{C}_2\text{H}_5 + \text{Br}_2 \rightarrow \text{C}_2\text{H}_5\text{Br} + \cdot \text{Br} \)
Termination:
\( \cdot \text{C}_2\text{H}_5 + \cdot \text{Br} \rightarrow \text{C}_2\text{H}_5\text{Br} \)
\( \cdot \text{C}_2\text{H}_5 + \cdot \text{C}_2\text{H}_5 \rightarrow \text{C}_4\text{H}_{10} \)
