IB DP Chemistry - R3.4.10 Rate of the substitution reactions - New Syllabus - 2026, 2027 & 2028
IB DP Chemistry – R3.4.10 Rate of the substitution reactions – Study Notes – New Syllabus
IITian Academy excellent Introduction to the Proton transfer reactions – Study Notes and effective strategies will help you prepare for your IB DP Chemistry exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 3.4.10 - The rate of the substitution reactions
Reactivity 3.4.10 – The rate of the substitution reactions
Leaving Group:
A leaving group is an atom or group of atoms that detaches from the carbon atom during a substitution reaction, taking with it the bonding electron pair. Its ability to depart easily is critical to the rate of the substitution reaction.
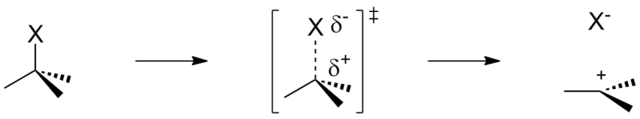
Key Factors Affecting the Leaving Group’s Ability:
- Bond Strength (C-X): The weaker the bond between the carbon and the halogen (X), the easier it is for the leaving group to depart. This enhances the rate of reaction.
- Polarity of the C-X Bond: A more polar bond (due to electronegativity differences) allows easier heterolytic cleavage of the bond during substitution.
- Stability of the Leaving Group: The more stable the leaving group is as an ion in solution, the more likely it is to leave. Larger halide ions are better at stabilizing negative charge.
- Size of the Halogen Atom: Larger halogens (e.g. I⁻) have more diffuse electron clouds and can stabilize charge better, making them better leaving groups.
Implication for Substitution Reactions:
In both SN1 and SN2 reactions, the bond between carbon and the leaving group must break. If the bond is very strong (as in the case of C-F), it becomes much harder for the reaction to occur. Thus, iodide (I⁻), being the most stable and having the weakest bond with carbon, is the best leaving group.
Relative rates of the substitution reactions for different halogenoalkanes
The rate of nucleophilic substitution reactions in halogenoalkanes is significantly affected by the identity of the halogen atom acting as the leaving group. The better the leaving group, the faster the substitution reaction occurs. Among the halogens, the relative order of leaving group ability is:
I⁻ > Br⁻ > Cl⁻ >> F⁻
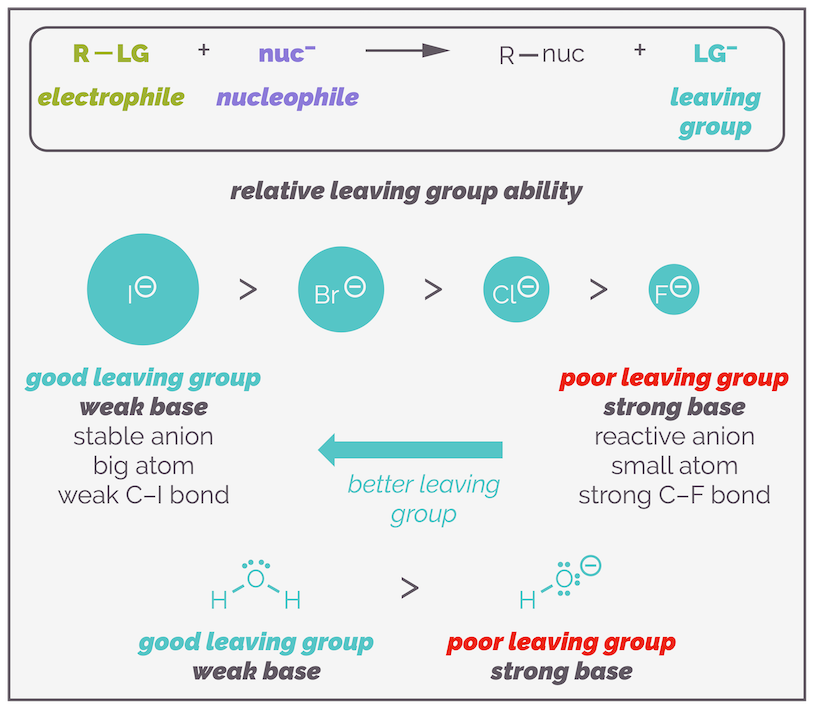
Key Reason:
- The bond strength between the carbon atom and the halogen (C-X bond) decreases down the group.
- As the halogen atom increases in size (from Cl to I), it forms a weaker bond with carbon, making it easier to break during substitution.
- Larger halide ions also stabilize the negative charge better when they depart, making them more effective leaving groups.
Example: Rate Comparison in SN1 or SN2 reactions
Suppose we compare the following halogenoalkanes under identical conditions:
- \( \text{CH}_3\text{CH}_2\text{Cl} \)
- \( \text{CH}_3\text{CH}_2\text{Br} \)
- \( \text{CH}_3\text{CH}_2\text{I} \)
The expected relative rate of reaction would be:
RI > RBr > RCl
This means that iodoethane undergoes nucleophilic substitution faster than bromoethane and much faster than chloroethane, due to the weaker C-I bond and better leaving group ability of I⁻.
Note:
- This trend applies regardless of whether the mechanism is SN1 or SN2.
- Fluoroalkanes are rarely reactive in substitution due to the very strong C-F bond (bond dissociation energy ≈ 485 kJ/mol).
Comparison of Common Halogen Substitutions:
| Halogen | Atomic Radius (pm) | C-X Bond Strength (kJ/mol) | Relative Rate of Substitution |
|---|---|---|---|
| F (Fluorine) | 64 | 485 | Very poor (C-F bond too strong) |
| Cl (Chlorine) | 99 | 338 | Moderate |
| Br (Bromine) | 114 | 276 | Good |
| I (Iodine) | 133 | 238 | Excellent |
Example
In a lab, equal volumes of 1-chlorobutane, 1-bromobutane, and 1-iodobutane are added separately to ethanolic silver nitrate. Which would show the fastest formation of a white/yellow precipitate of silver halide? Justify your answer.
▶️Answer/Explanation
Answer: 1-iodobutane reacts the fastest and forms a yellow precipitate of AgI.
Explanation:
The reaction proceeds via nucleophilic substitution where the halogen is replaced, and the halide ion reacts with Ag⁺ to form a precipitate:
\( \text{R–X} + \text{Ag}^+ \rightarrow \text{AgX} \downarrow + \text{R}^+ \)
The leaving group ability increases in the order: Cl⁻ < Br⁻ < I⁻
Iodide has the weakest bond with carbon and forms the most stable ion, so 1-iodobutane shows the fastest reaction and forms AgI the quickest.
Example
Rank the following halogenoalkanes in order of increasing rate of nucleophilic substitution and explain:
\( \text{CH}_3\text{CH}_2\text{Cl}, \text{CH}_3\text{CH}_2\text{Br}, \text{CH}_3\text{CH}_2\text{I} \)
▶️Answer/Explanation
Ranking:
\( \text{CH}_3\text{CH}_2\text{Cl} \lt \text{CH}_3\text{CH}_2\text{Br} \lt \text{CH}_3\text{CH}_2\text{I} \)
Explanation:
The rate of substitution depends on how easily the C–X bond breaks and how stable the leaving group is.
- C–Cl bond is strongest and Cl⁻ is the poorest leaving group
- C–I bond is weakest and I⁻ is the best leaving group
Therefore, ethyl iodide reacts fastest, followed by ethyl bromide, and ethyl chloride is the slowest.
Example
A student observes the following order of reaction rates with NaOH (aq):
\( \text{CH}_3\text{CH}_2\text{I} \gt \text{CH}_3\text{CH}_2\text{Br} \gt \text{CH}_3\text{CH}_2\text{Cl} \).
Explain this trend in terms of bond enthalpy and leaving group stability.
▶️Answer/Explanation
Explanation:
- The C–I bond has the lowest bond enthalpy (~213 kJ/mol), making it easiest to break.
- The iodide ion (I⁻) is large and stabilizes negative charge well, acting as a very good leaving group.
- In contrast, the C–Cl bond is strongest (~338 kJ/mol) and Cl⁻ is a poor leaving group.
So, the observed trend reflects both weaker C–X bond and greater stability of the leaving group as you move from Cl to I.
Leaving Group:
A leaving group is an atom or group of atoms that detaches from the carbon atom during a substitution reaction, taking with it the bonding electron pair. Its ability to depart easily is critical to the rate of the substitution reaction.
Key Factors Affecting the Leaving Group’s Ability:
- Bond Strength (C-X): The weaker the bond between the carbon and the halogen (X), the easier it is for the leaving group to depart. This enhances the rate of reaction.
- Polarity of the C-X Bond: A more polar bond (due to electronegativity differences) allows easier heterolytic cleavage of the bond during substitution.
- Stability of the Leaving Group: The more stable the leaving group is as an ion in solution, the more likely it is to leave. Larger halide ions are better at stabilizing negative charge.
- Size of the Halogen Atom: Larger halogens (e.g. I⁻) have more diffuse electron clouds and can stabilize charge better, making them better leaving groups.
Implication for Substitution Reactions:
In both SN1 and SN2 reactions, the bond between carbon and the leaving group must break. If the bond is very strong (as in the case of C-F), it becomes much harder for the reaction to occur. Thus, iodide (I⁻), being the most stable and having the weakest bond with carbon, is the best leaving group.
Example
Explain why iodoalkanes undergo nucleophilic substitution reactions more rapidly than chloroalkanes.
▶️Answer/Explanation
The C-I bond is longer and weaker than the C-Cl bond, making it easier to break. Iodide (I⁻) is a much better leaving group than chloride (Cl⁻) due to its larger size and lower charge density. Therefore, iodoalkanes react faster in substitution reactions.
Example
Rank the leaving groups Cl⁻, Br⁻, and I⁻ in terms of their effectiveness in promoting nucleophilic substitution reactions. Justify your answer with reference to bond strength and stability.
▶️Answer/Explanation
Order: Cl⁻ < Br⁻ < I⁻
The bond strength decreases from C-Cl to C-I. Since the C-I bond is the weakest, it breaks most easily. Also, I⁻ is the most stable leaving group due to its larger atomic radius, making iodo compounds the most reactive in substitution reactions.
Relative rates of the substitution reactions for different halogenoalkanes
The rate of nucleophilic substitution reactions in halogenoalkanes is significantly affected by the identity of the halogen atom acting as the leaving group. The better the leaving group, the faster the substitution reaction occurs. Among the halogens, the relative order of leaving group ability is:
I⁻ > Br⁻ > Cl⁻ >> F⁻
Key Reason:
- The bond strength between the carbon atom and the halogen (C-X bond) decreases down the group.
- As the halogen atom increases in size (from Cl to I), it forms a weaker bond with carbon, making it easier to break during substitution.
- Larger halide ions also stabilize the negative charge better when they depart, making them more effective leaving groups.
Example: Rate Comparison in SN1 or SN2 reactions
Suppose we compare the following halogenoalkanes under identical conditions:
- \( \text{CH}_3\text{CH}_2\text{Cl} \)
- \( \text{CH}_3\text{CH}_2\text{Br} \)
- \( \text{CH}_3\text{CH}_2\text{I} \)
The expected relative rate of reaction would be:
RI > RBr > RCl
This means that iodoethane undergoes nucleophilic substitution faster than bromoethane and much faster than chloroethane, due to the weaker C-I bond and better leaving group ability of I⁻.
Note:
- This trend applies regardless of whether the mechanism is SN1 or SN2.
- Fluoroalkanes are rarely reactive in substitution due to the very strong C-F bond (bond dissociation energy ≈ 485 kJ/mol).
Comparison of Common Halogens:
| Halogen | Atomic Radius (pm) | C-X Bond Strength (kJ/mol) | Relative Rate of Substitution |
|---|---|---|---|
| F (Fluorine) | 64 | 485 | Very poor (C-F bond too strong) |
| Cl (Chlorine) | 99 | 338 | Moderate |
| Br (Bromine) | 114 | 276 | Good |
| I (Iodine) | 133 | 238 | Excellent |
Example
Arrange the following halogenoalkanes in increasing order of reactivity towards a nucleophile in an SN2 reaction:
\( \text{CH}_3\text{CH}_2\text{Cl}, \text{CH}_3\text{CH}_2\text{Br}, \text{CH}_3\text{CH}_2\text{I} \)
▶️Answer/Explanation
Order of reactivity:
\( \text{CH}_3\text{CH}_2\text{Cl} < \text{CH}_3\text{CH}_2\text{Br} < \text{CH}_3\text{CH}_2\text{I} \)
Iodoethane has the weakest C-I bond and forms the most stable I⁻ leaving group, making it the fastest to react. Chloroethane has the strongest C-Cl bond and reacts the slowest.
Example
Which compound would undergo a faster nucleophilic substitution reaction under identical conditions: iodoethane or bromoethane? Explain your answer.
▶️Answer/Explanation
Answer: Iodoethane
Explanation: The C-I bond in iodoethane is weaker than the C-Br bond in bromoethane (238 kJ/mol vs. 276 kJ/mol), so it requires less energy to break. I⁻ is also a better leaving group due to its larger size and better charge delocalization, making iodoethane react faster.
