IB DP Chemistry -R3.4.4 Electrophiles - Study Notes - New Syllabus - 2026, 2027 & 2028
IB DP Chemistry – R2.2.13 Arrhenius factor – Study Notes – New Syllabus
IITian Academy excellent Introduction to the Proton transfer reactions – Study Notes and effective strategies will help you prepare for your IB DP Chemistry exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 3.4.4 — Electrophiles
Reactivity 3.4.4 — Electrophiles
An electrophile is a species that seeks electrons and accepts a pair of electrons from a nucleophile to form a covalent bond. Electrophiles are attracted to regions of high electron density (such as double bonds or lone pairs).
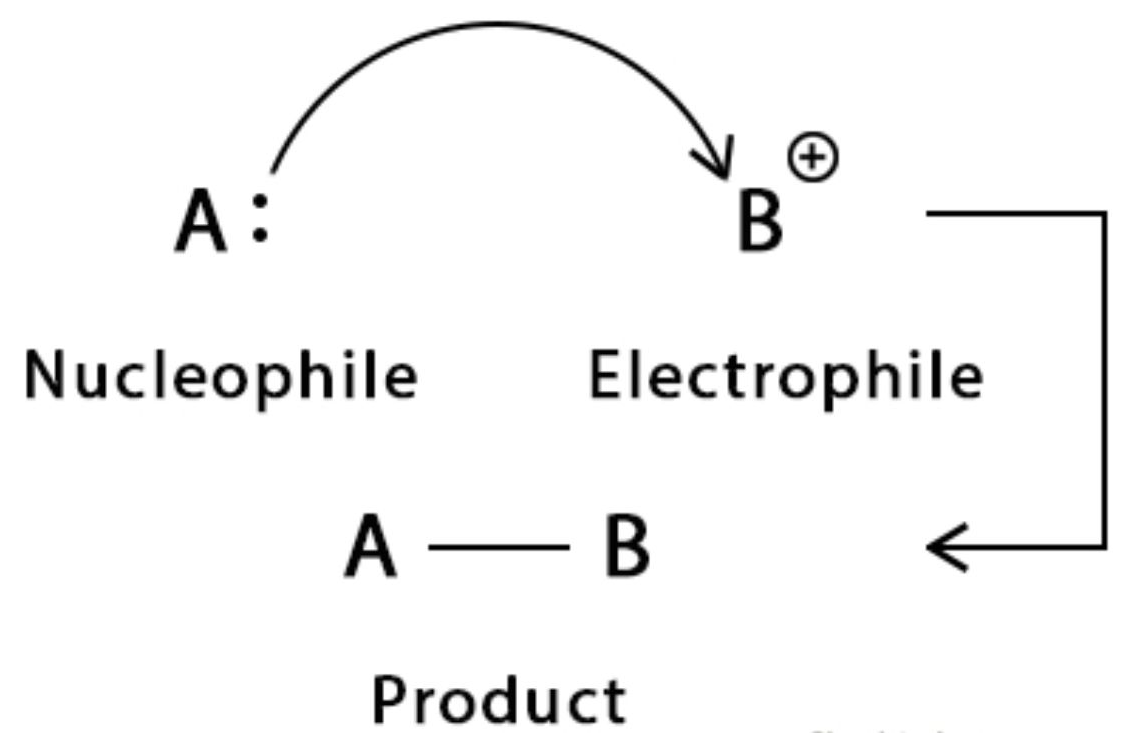
In essence, electrophiles are electron pair acceptors. This concept is consistent with the Lewis definition of acids (electrophiles are Lewis acids).
Key Characteristics of Electrophiles:
- Have a partial positive charge (\( \delta^+ \)) or full positive charge (\( ^+ \))
- Can be atoms, ions, or molecules
- Often have an incomplete octet or an atom with a low electron density
- Participate in addition or substitution mechanisms, especially in organic chemistry
General Mechanism Representation:
Curly arrows are used to represent the movement of an electron pair from the nucleophile to the electrophile. The arrow starts from the electron-rich center and points toward the electron-deficient atom.
\( \text{Nucleophile} \ + \ \text{Electrophile} \longrightarrow \text{New compound} \)
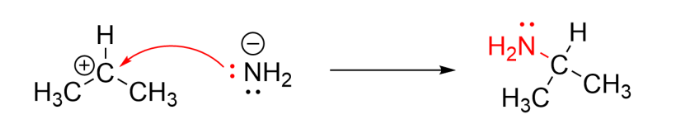
Examples of Electrophiles:
| Electrophile | Charge | Reaction Type | Reason for Electrophilicity |
|---|---|---|---|
| \( \text{H}^+ \) | Positive | Acid-base | Needs two electrons to form a bond |
| \( \text{NO}_2^+ \) | Positive | Electrophilic substitution | Electron-deficient nitrogen |
| \( \text{Br}_2 \) | Neutral | Electrophilic addition | Becomes polarized in presence of double bond |
| \( \text{AlCl}_3 \) | Neutral | Electrophilic substitution (catalyst) | Electron-deficient Al center |
| \( \text{CH}_3^+ \) | Positive | Nucleophilic substitution | Carbocation; incomplete octet |
Common Electrophilic Reaction Types:
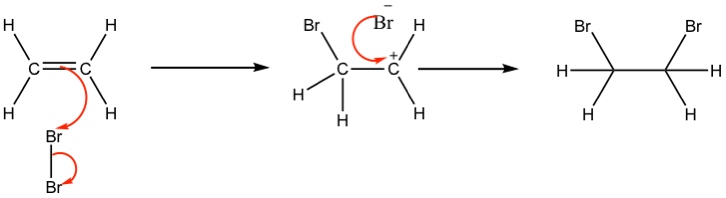
Electrophilic addition: Electrophile attacks a C=C double bond (e.g. bromine to ethene)
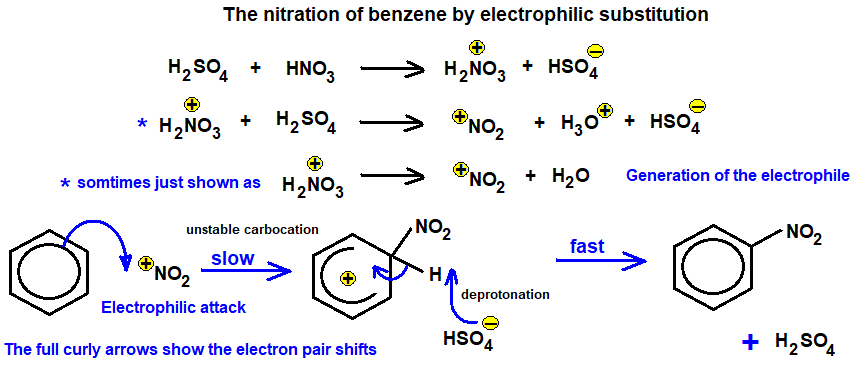
Electrophilic substitution: Occurs in aromatic rings (e.g. nitration of benzene)
Acid-base reactions: Proton (\( \text{H}^+ \)) acts as electrophile
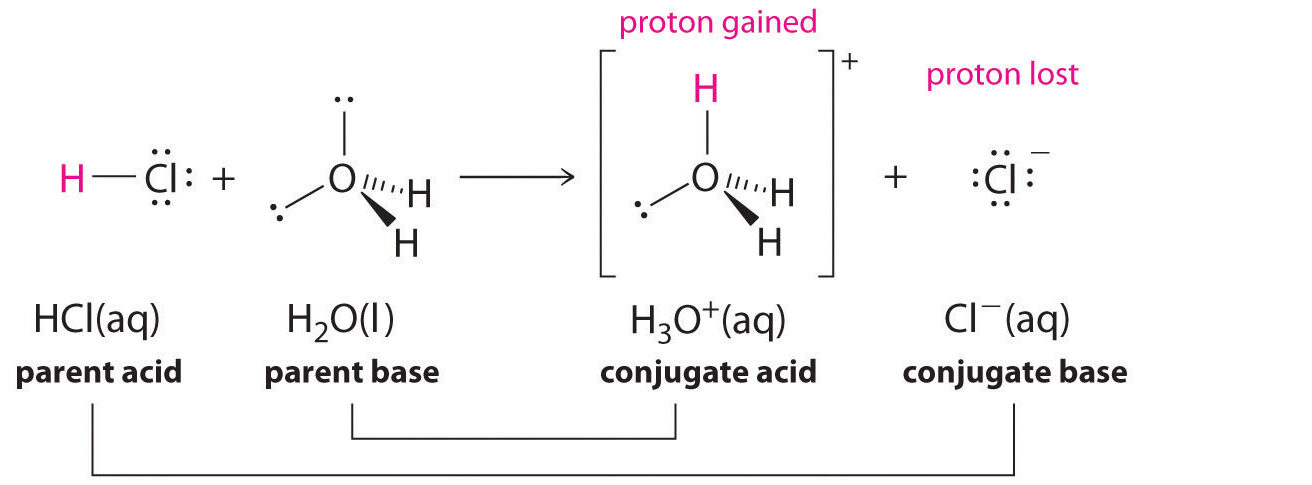
Example
Bromine reacts with ethene in an electrophilic addition reaction. Identify the electrophile and show how the reaction proceeds.
▶️Answer/Explanation
Ethene has a region of high electron density (π bond). The approaching \( \text{Br}_2 \) molecule becomes polarized:
\( \text{Br}-\delta^+ \cdots \text{Br}-\delta^- \)
The double bond donates electrons to the \( \text{Br}^\delta^+ \), forming a cyclic bromonium ion intermediate and a \( \text{Br}^- \) ion.
\( \text{CH}_2=CH_2 + \text{Br}_2 \rightarrow \text{CH}_2Br-CH_2Br \)
Thus, \( \text{Br}_2 \) acts as the electrophile by accepting electrons from the alkene.
Example
In the nitration of benzene, identify the electrophile and show how it is generated.
▶️Answer/Explanation
The electrophile is the nitronium ion (\( \text{NO}_2^+ \)), generated from the reaction:
\( \text{HNO}_3 + \text{H}_2\text{SO}_4 \rightarrow \text{NO}_2^+ + \text{HSO}_4^- + \text{H}_2\text{O} \)
This ion is highly electron-deficient and reacts with the π-electrons in benzene in an electrophilic substitution reaction.
Example
Identify the electrophile in the hydrolysis of tert-butyl chloride (\( (CH_3)_3CCl \)) in water.
▶️Answer/Explanation
The mechanism follows an SN1 pathway:
\( (CH_3)_3CCl \rightarrow (CH_3)_3C^+ + Cl^- \)
The water molecule (nucleophile) donates a lone pair to the carbocation. Here, the carbocation is the electrophile because it accepts the electron pair.
Key Points:
- Electrophiles are often positively charged or electron-deficient.
- Recognizing electrophiles helps predict reaction pathways, especially in substitution and addition mechanisms.
- Curly arrows must always show the flow of electrons from nucleophile to electrophile.
- Knowledge of electrophilic species is essential for understanding acid-base theory, organic mechanisms, and coordination chemistry.
