IB DP Chemistry - S1.3.7 Successive ionization energies - Study Notes - New Syllabus - 2026, 2027 & 2028
IB DP Chemistry – S1.3.7 Successive ionization energies – Study Notes – New Syllabus
IITian Academy excellent Study Notes and effective strategies will help you prepare for your IB DP Chemistry exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Structure 1.3.7 — Successive Ionization Energies and Electron Configuration
Structure 1.3.7 — Successive Ionization Energies and Electron Configuration
Successive Ionization Energies
Successive ionization energies refer to the amount of energy required to remove electrons one by one from a single atom in the gaseous state.
- 1st IE: Energy to remove the first electron.
- 2nd IE: Energy to remove the second electron.
- 3rd IE: Energy to remove the third electron, and so on.
Units: Usually expressed in kJ mol⁻¹.
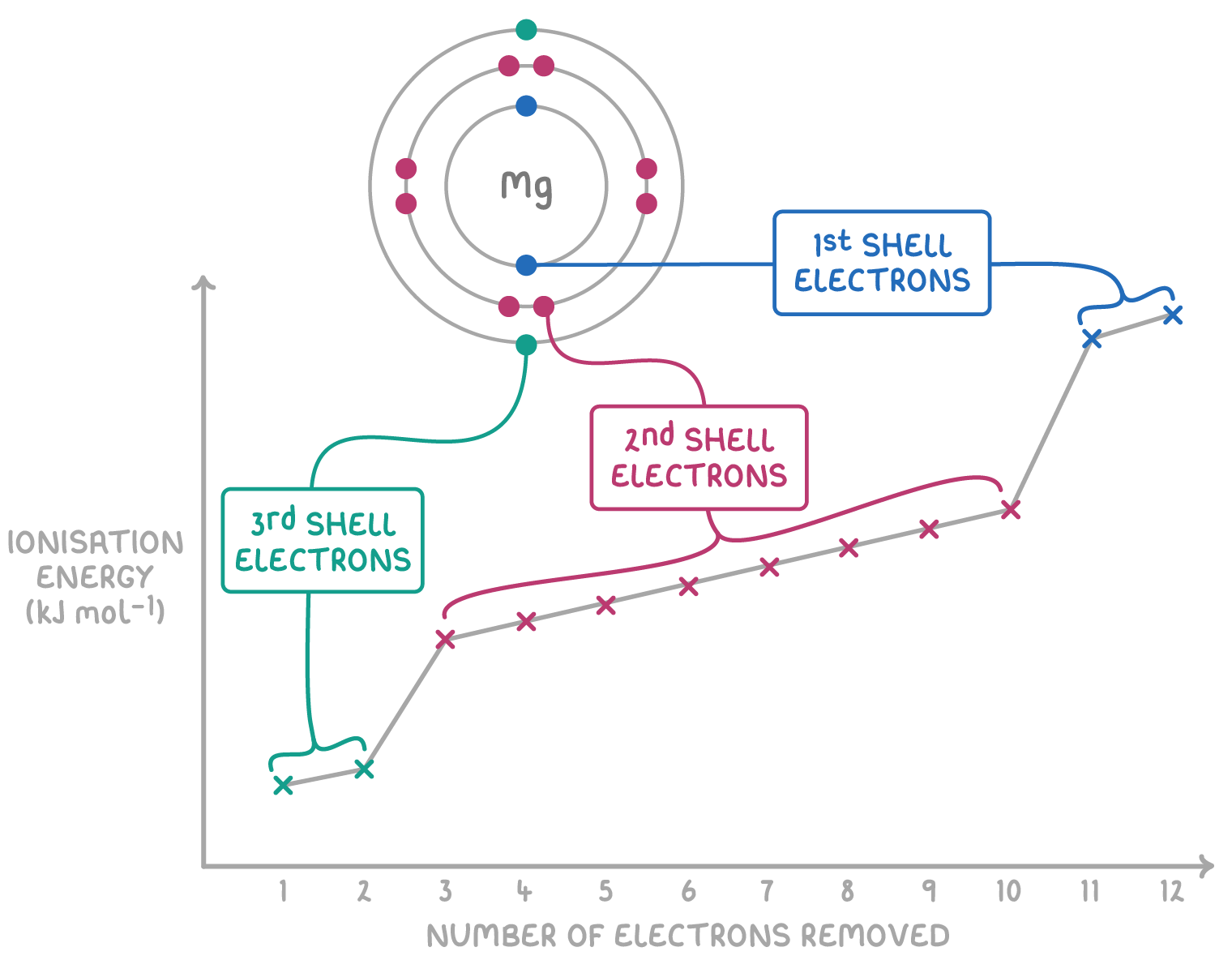
Observation: Ionization energy increases with each successive electron removed because:
- Electrons are removed from an increasingly positive ion.
- Less shielding and greater attraction to the nucleus.
Big Jumps in Ionization Energies
A large increase between two successive ionization energies suggests that the electron removed comes from a new (inner) energy level.
This jump is used to determine the group number of the element.
Strategy to Deduce Group Number:
- List successive IEs.
- Look for the large jump — this occurs after the outer electrons are removed.
- The number of electrons removed before the large jump = group number (for main group elements).
Example:
Successive Ionization Energy Data (in kJ mol⁻¹)
| Ionization Number | 1st IE | 2nd IE | 3rd IE | 4th IE |
|---|---|---|---|---|
| Energy (kJ mol⁻¹) | 577 | 1812 | 2740 | 11,600 |
Explanation: There’s a large jump after the 3rd ionization energy. This suggests that 3 valence electrons were relatively easy to remove, and the 4th is from a more stable inner shell.
Deduction: The element has 3 valence electrons → belongs to Group 13 (e.g., aluminium).
Tip: Plotting IE vs. number of electrons removed helps visually detect where the big jump occurs.
Example :
Deducing the Group Number
Given the successive ionization energies (in kJ mol⁻¹):
| 1st IE | 2nd IE | 3rd IE | 4th IE |
|---|---|---|---|
| 495 | 4560 | 6910 | 9540 |
▶️Answer/Explanation
The large jump is between the 1st and 2nd ionization energy.
This means only 1 valence electron was present — removing the second requires breaking into an inner shell.
Conclusion: The element is in Group 1, like sodium (Na).
Example :
Identifying the Group from a Jump
Successive IEs (kJ mol⁻¹):
| 1st IE | 2nd IE | 3rd IE | 4th IE | 5th IE |
|---|---|---|---|---|
| 738 | 1450 | 7730 | 10500 | 13600 |
▶️Answer/Explanation
There’s a sharp increase between the 2nd and 3rd ionization energy.
So, 2 electrons are in the outer shell before the big jump.
Conclusion: The element is in Group 2, such as magnesium (Mg).
Example :
Determining Electron Configuration
Given ionization energies (kJ mol⁻¹):
| 1st IE | 2nd IE | 3rd IE | 4th IE |
|---|---|---|---|
| 900 | 1750 | 14800 | 21000 |
▶️Answer/Explanation
Large jump is after the 2nd IE → two valence electrons were removed easily, third requires core-level removal.
Electron configuration likely ends in: \( ns^2 \)
Element likely to be: Group 2 (e.g., Be, Mg, Ca).
Example :
Deducing Group Number from Ionization Energy Graph
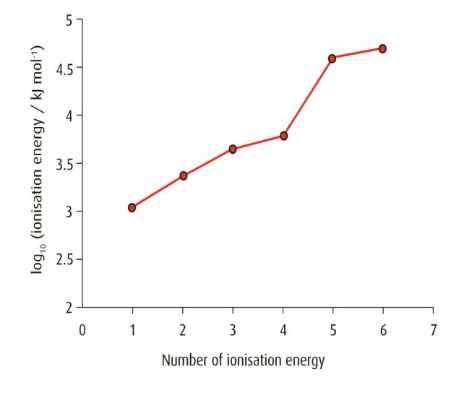
▶️Answer/Explanation
The element belongs to Group 4.
There is a noticeable jump in ionization energy between the 4th and 5th electrons. This suggests the first four electrons are in the outer shell, and the 5th is from an inner shell.
Removing an inner electron requires significantly more energy because it’s closer to the nucleus and experiences stronger electrostatic attraction.
Example :
Deducing Group Number from Ionization Energy Graph
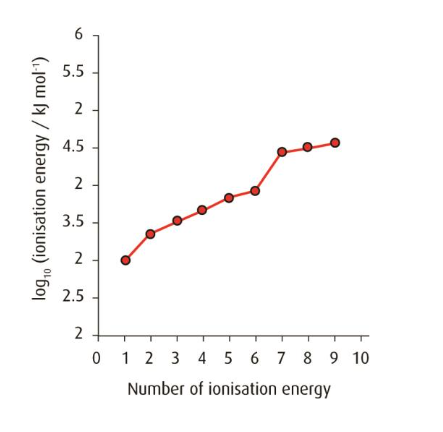
▶️Answer/Explanation
The element belongs to Group 6.
A large increase in ionization energy occurs between the 6th and 7th electrons, showing that the first six electrons are outer electrons.
The 7th electron is being removed from a new, inner energy level—closer to the nucleus—so it requires much more energy to remove due to stronger nuclear attraction.
