IB DP Chemistry - S2.2.1 Formation of covalent bonds- Study Notes - New Syllabus - 2026, 2027 & 2028
IB DP Chemistry – S2.2.1 Formation of covalent bonds – Study Notes – New Syllabus
IITian Academy excellent Study Notes and effective strategies will help you prepare for your IB DP Chemistry exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Structure 2.2.1 — Covalent Bonding and the Octet Rule
Structure 2.2.1 — Covalent Bonding and the Octet Rule
A covalent bond is formed when two atoms share a pair of electrons. The bond arises due to the electrostatic attraction between the shared pair of electrons and the positively charged nuclei of both atoms.
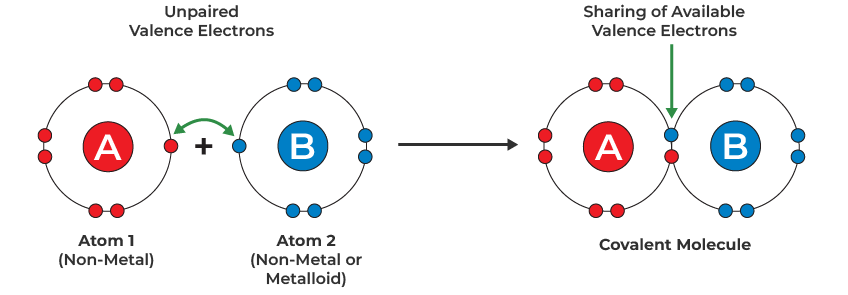
Features of Covalent Bonding:
- Occurs between non-metal atoms.
- Electrons are shared, not transferred.
- The goal is for each atom to achieve a stable electron configuration (usually a full outer shell).
- Can involve single, double or triple bonds depending on how many pairs are shared.
The Octet Rule:
The Octet Rule states that atoms tend to form bonds in such a way that each atom (except for a few exceptions) ends up with eight electrons in its valence shell, which corresponds to a full s and p subshell—like the stable configuration of a noble gas.
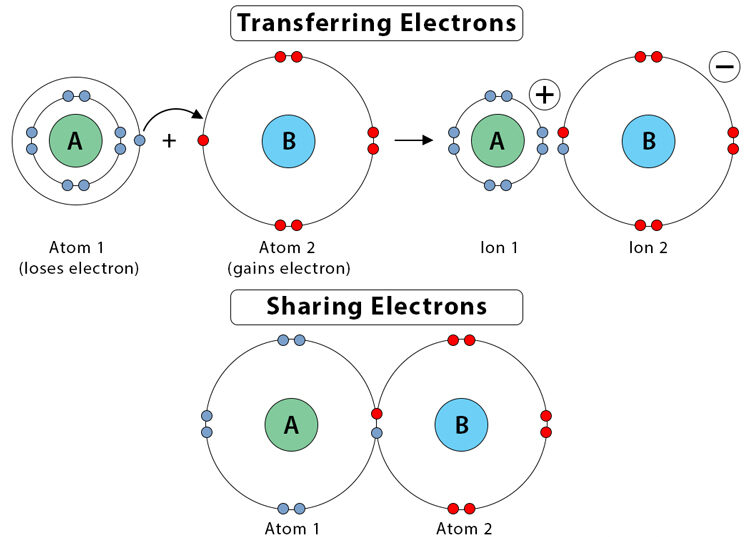
Application in Lewis Structures:
- Atoms share electrons through covalent bonding so that each atom achieves a noble gas configuration.
- Each bond represents a shared pair of electrons (one from each atom).
- Non-bonding electrons (lone pairs) are shown as pairs of dots or dashes.
Common Octet Rule Followers:
- Carbon (C) – always forms 4 bonds
- Nitrogen (N) – typically forms 3 bonds, with 1 lone pair
- Oxygen (O) – typically forms 2 bonds, with 2 lone pairs
- Halogens (Cl, Br, etc.) – usually form 1 bond, with 3 lone pairs
Exceptions to the Octet Rule:
Incomplete Octet: Some atoms are stable with fewer than 8 electrons:
- Hydrogen (H) – stable with 2 electrons (1 bond)
- Beryllium (Be) – stable with 4 electrons (often 2 bonds)
- Boron (B) – stable with 6 electrons (usually 3 bonds)
Expanded Octet: Elements in Period 3 or higher can accommodate more than 8 electrons in their valence shell due to the availability of empty d orbitals. Examples:
- Phosphorus (P) in \( \text{PCl}_5 \) has 10 valence electrons
- Sulfur (S) in \( \text{SF}_6 \) has 12 valence electrons
Odd-Electron Molecules: Molecules like \( \text{NO} \) or \( \text{NO}_2 \) have an odd number of electrons and cannot distribute them to give every atom an octet.
These exceptions must be taken into account when drawing Lewis structures especially for elements in Groups 2 and 13, or Period 3 and beyond.
Examples of Simple Covalent Molecules:
- Hydrogen molecule: \( \text{H}_2 \) — each H shares 1 electron → duet configuration.
- Chlorine molecule: \( \text{Cl}_2 \) — each Cl shares 1 electron → octet configuration.
- Water molecule: \( \text{H}_2\text{O} \) — O shares 2 electrons, each H shares 1.
- Methane: \( \text{CH}_4 \) — C shares 4 electrons with 4 H atoms → octet for C.
Example
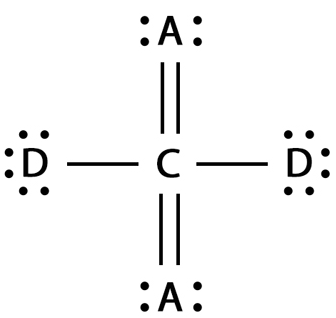
Explain the covalent bonding in a molecule of carbon dioxide, \( \text{CO}_2 \). Explain how the octet rule is satisfied for each atom.
▶️Answer/Explanation
Carbon has 4 valence electrons, and each oxygen atom has 6. In \( \text{CO}_2 \), carbon forms two double bonds, one with each oxygen atom. Each double bond involves 2 shared pairs of electrons.
By sharing 4 electrons (2 pairs with each O), carbon achieves 8 valence electrons (octet). Each oxygen atom also shares 2 electrons with carbon, gaining a full octet (6 from itself + 2 shared).
Example
Explain why the molecule \( \text{BF}_3 \) does not follow the octet rule.
▶️Answer/Explanation
Boron has 3 valence electrons. Each fluorine atom contributes 1 electron to form a single covalent bond with boron.
In \( \text{BF}_3 \), boron forms 3 single covalent bonds with 3 fluorine atoms, leading to a total of 6 electrons around boron—this is an incomplete octet.
Fluorine atoms, however, each have 6 non-bonding electrons + 2 bonding electrons, so they obey the octet rule.
This is an exception to the octet rule—boron is electron-deficient.
Lewis Structures of Molecules and Ions
A Lewis structure (also known as an electron dot diagram) represents the valence electrons in a molecule or polyatomic ion. It shows how atoms are bonded together and the placement of bonding pairs and lone pairs of electrons.
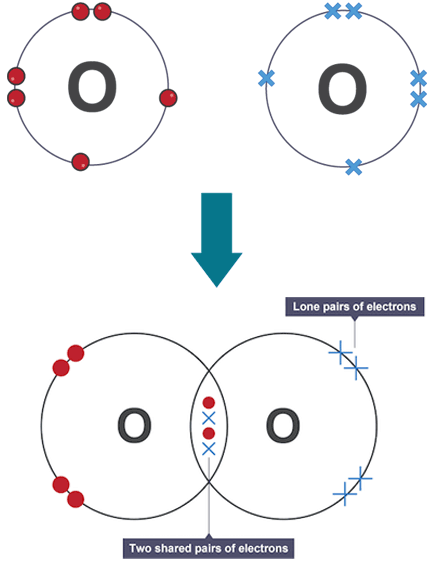
Key Notes about Lewis Structures :
- Only valence electrons are shown in Lewis structures.
- Bonding electrons (shared between atoms) are usually shown as lines (–) or as a pair of dots.
- Non-bonding electrons (lone pairs) are shown as pairs of dots or crosses around atoms.
- Each bond (single, double, triple) corresponds to 1, 2, or 3 shared pairs of electrons.
Step-by-Step Method for Drawing Lewis Structures:
- Count total valence electrons in the molecule/ion.
- Add electrons for each atom (from the periodic table).
- For anions, add extra electrons equal to the negative charge.
- For cations, subtract electrons equal to the positive charge.
- Choose the central atom (usually the least electronegative, except H and F).
- Place single bonds between the central atom and surrounding atoms.
- Distribute remaining electrons to complete octets (or duets for H), placing lone pairs on outer atoms first, then the central atom.
- Use multiple bonds (double or triple) if necessary to satisfy the octet rule.
Charge in Polyatomic Ions:
- Brackets \([ ]\) are used around polyatomic ion structures.
- The total charge is written as a superscript outside the brackets: e.g., \([ \text{NH}_4 ]^+ \)
Formal Charge (IBDP-AHL):
Formal charge is used to evaluate the most likely Lewis structure when more than one is possible.
Formula:
\(\text{Formal Charge} = \text{(Valence electrons)} – \text{(Non-bonding electrons)} – \frac{1}{2} \times \text{(Bonding electrons)} \)
Notes:
- The best Lewis structure has formal charges as close to 0 as possible.
- Negative formal charges are preferably placed on more electronegative atoms.
Example
Draw the Lewis structure of \( \text{CH}_2\text{O} \) (formaldehyde). Include all lone pairs and verify the octet rule.
▶️Answer/Explanation
Carbon has 4 valence electrons, oxygen has 6, and each hydrogen has 1.
Total valence electrons = \( 4 + 2(1) + 6 = 12 \)
Carbon forms a double bond with oxygen and single bonds with each hydrogen.
Structure: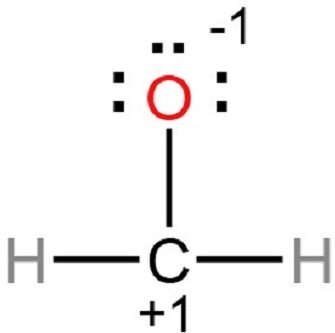
Oxygen has two lone pairs, carbon has 4 bonds = octet satisfied.
All atoms obey the octet rule.
Example
Draw the Lewis structure for \( \text{BF}_3 \). Does the central atom follow the octet rule?
▶️Answer/Explanation
Boron has 3 valence electrons, fluorine has 7 each.
Total valence electrons = \( 3 + 3(7) = 24 \)
Structure:
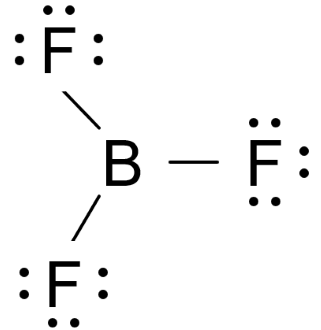
Each fluorine has 3 lone pairs.
Boron has only 6 electrons – this is an incomplete octet.
This is acceptable for boron (Group 13).
Example
Draw the Lewis structure of the sulfate ion \( \text{SO}_4^{2-} \). Does sulfur obey the octet rule?
▶️Answer/Explanation
Sulfur has 6 valence electrons, each oxygen has 6, and we add 2 for the charge.
Total = \( 6 + 4(6) + 2 = 32 \) electrons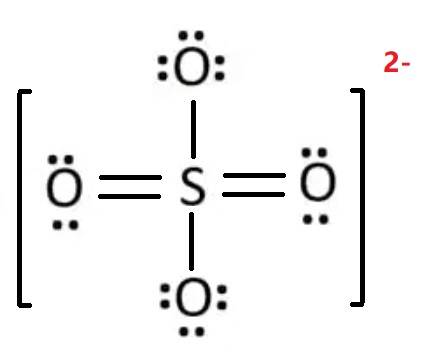
Sulfur has 12 electrons around it → an expanded octet.
Allowed for elements in Period 3 and beyond.
Example
Draw the Lewis structure of \( \text{NO}_2 \). Explain why it violates the octet rule.
▶️Answer/Explanation
Nitrogen has 5 electrons, each oxygen has 6 → total = \( 5 + 2(6) = 17 \) electrons.
This is an odd-electron species.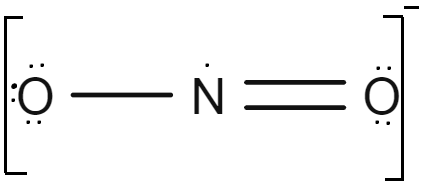
Nitrogen ends up with only 7 electrons → does not follow the octet rule.
Resonance structures exist, but the odd number of electrons makes this an exception.
Example
Draw the Lewis structure of \( \text{PCl}_5 \). Does phosphorus follow the octet rule?
▶️Answer/Explanation
Phosphorus has 5 valence electrons, chlorine has 7 each.
Total = \( 5 + 5(7) = 40 \) electrons.
Structure:
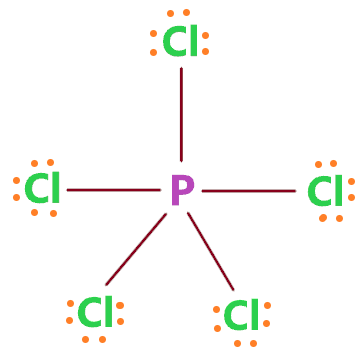
This is an expanded octet, allowed for Period 3 elements like P.
Example
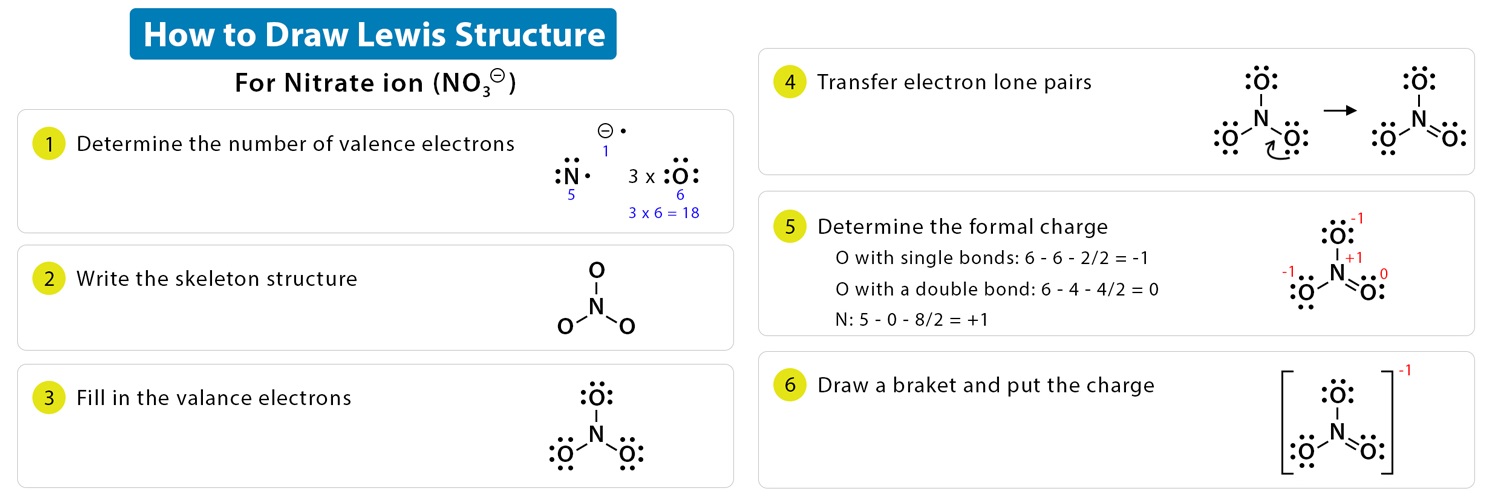
Determine the formal charge on each atom in the Lewis structure of the nitrate ion \( \text{NO}_3^- \).
▶️Answer/Explanation
Choose a valid Lewis structure
One common structure of \( \text{NO}_3^- \) has nitrogen double bonded to one oxygen, and single bonded to the other two oxygen atoms, each carrying a negative charge.
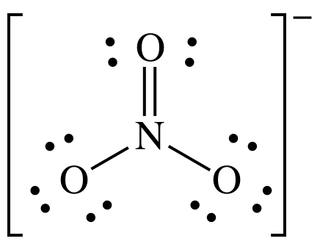
Apply formal charge formula:
$ \text{Formal charge} = \text{valence electrons} – \text{non-bonding electrons} – \frac{1}{2} \times \text{bonding electrons} $
- Nitrogen (N): valence = 5, lone pairs = 0, bonding electrons = 8 (4 bonds) $ \text{FC} = 5 – 0 – \frac{8}{2} = 5 – 4 = +1 $
- Double-bonded oxygen (O): valence = 6, lone pairs = 4, bonding electrons = 4 $ \text{FC} = 6 – 4 – \frac{4}{2} = 6 – 4 – 2 = 0 $
- Each single-bonded oxygen (O–): valence = 6, lone pairs = 6, bonding electrons = 2 $ \text{FC} = 6 – 6 – \frac{2}{2} = 6 – 6 – 1 = -1 $
Total formal charge: \( +1 + (-1) + (-1) = -1 \)
matches the charge on \( \text{NO}_3^- \)
