IB DP Chemistry - S2.2.10 Chromatography- Study Notes - New Syllabus - 2026, 2027 & 2028
IB DP Chemistry – S2.2.10 Chromatography- Study Notes – New Syllabus
IITian Academy excellent Study Notes and effective strategies will help you prepare for your IB DP Chemistry exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Structure 2.2.10 — Chromatography and Intermolecular Forces
Structure 2.2.10 — Chromatography and Intermolecular Forces
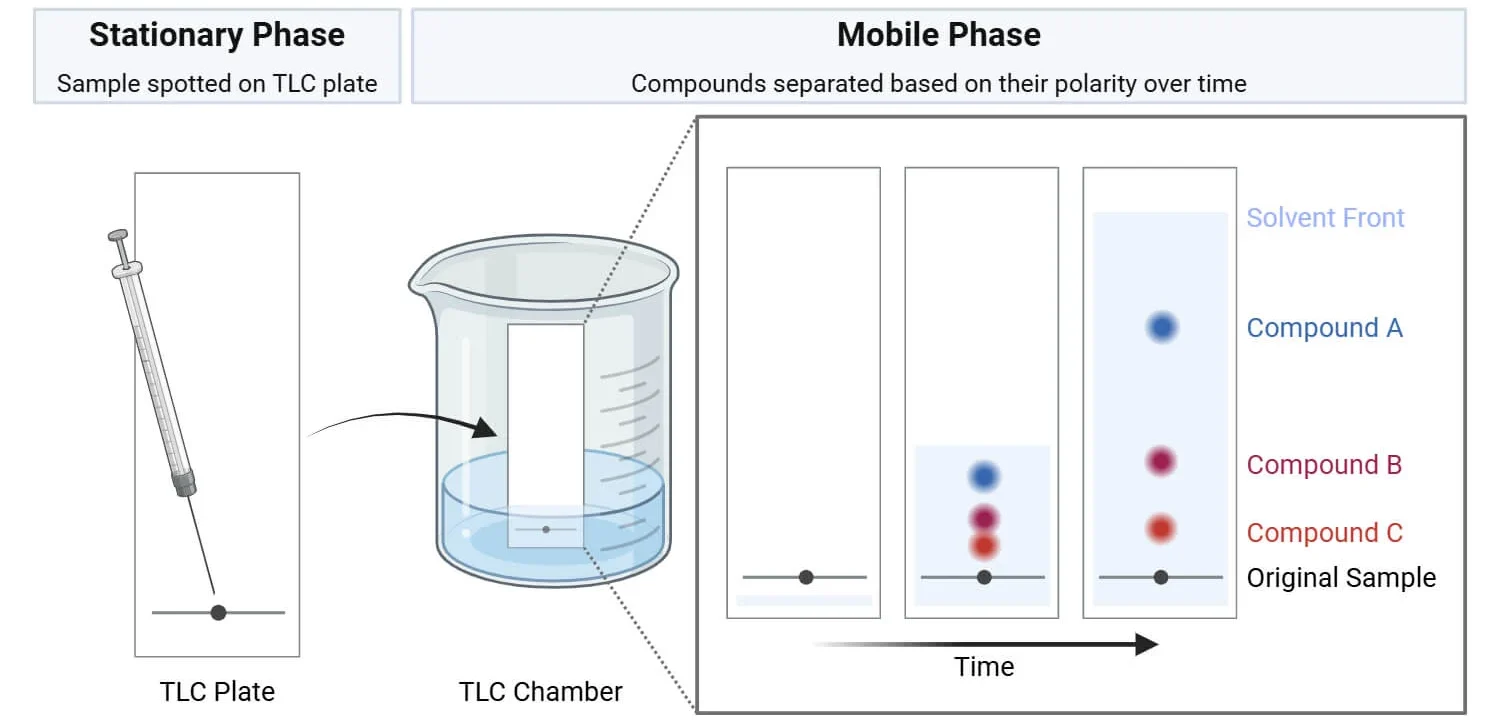
Chromatography is a physical separation technique used to separate the components of a mixture based on their relative attractions to the mobile phase and the stationary phase.
These attractions depend on the nature and strength of intermolecular forces such as hydrogen bonding, dipole–dipole interactions, and London dispersion forces.
Phases:
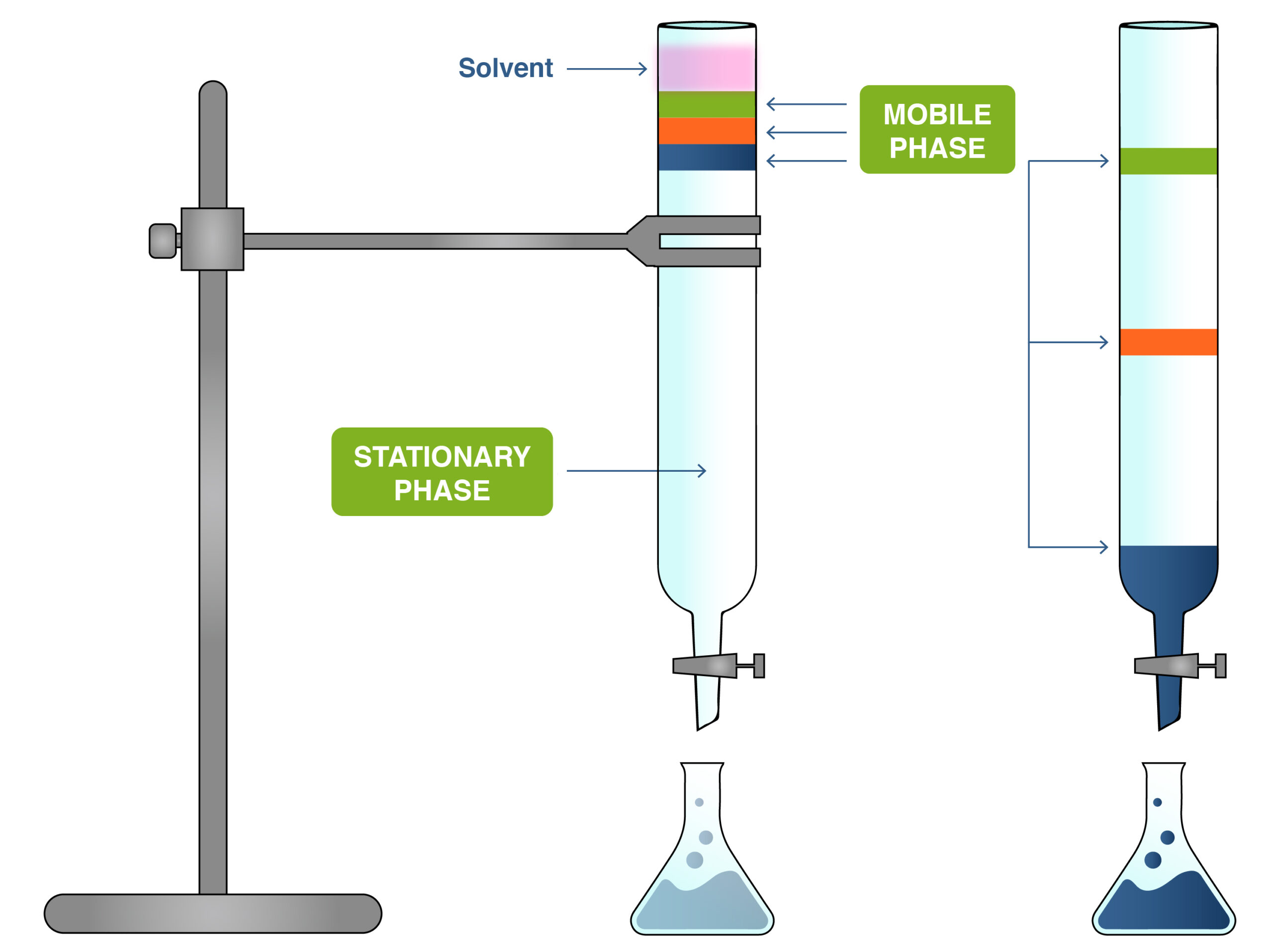
- Mobile phase: The solvent that moves through the stationary phase and carries the components of the mixture.
- Stationary phase: A fixed material (e.g. paper, silica gel) that interacts with the substances via intermolecular forces.
Principle of Separation:
- Substances that are more attracted to the mobile phase travel further.
- Substances that are more attracted to the stationary phase travel shorter distances.
- The degree of interaction is determined by the polarity and strength of intermolecular forces between the substances and the two phases.
Intermolecular Force Involvement:
- Polar molecules tend to interact strongly with polar stationary phases (e.g. silica gel) via hydrogen bonds or dipole–dipole forces.
- Non-polar molecules interact more with non-polar solvents (mobile phase) via dispersion forces.
Retardation Factor (\( R_f \)):
The retardation factor is a quantitative measure of how far a substance has travelled relative to the solvent front. It is calculated using:
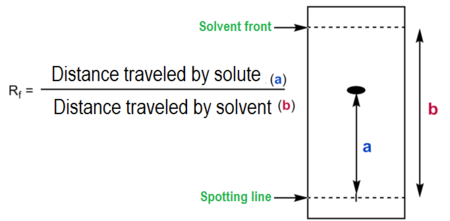
\( R_f = \dfrac{\text{distance travelled by solute}}{\text{distance travelled by solvent front}} \)
Key Points:
- \( R_f \) values are always between 0 and 1.
- A larger \( R_f \) value means the substance moved more with the solvent (greater attraction to mobile phase).
- A smaller \( R_f \) means the substance was retained more strongly by the stationary phase (stronger intermolecular interactions with it).
- \( R_f \) values are specific to the solvent system used.
Example
A student spots a black ink sample on chromatography paper. After running the paper in water, a blue dye moves 2.8 cm from the baseline while the solvent front moves 5.6 cm.
▶️ Answer/Explanation
Use the formula:
\( R_f = \dfrac{\text{distance travelled by solute}}{\text{distance travelled by solvent front}} \)
\( R_f = \dfrac{2.8}{5.6} = 0.50 \)
The \( R_f \) value is 0.50, meaning the dye has moderate attraction to both the stationary and mobile phases.
Example
In a chromatographic separation using ethanol as the mobile phase, a yellow pigment moves 3.2 cm while the solvent front moves 8.0 cm. Another pigment only moves 1.2 cm.
▶️ Answer/Explanation
For the yellow pigment:
\( R_f = \dfrac{3.2}{8.0} = 0.40 \)
For the second pigment:
\( R_f = \dfrac{1.2}{8.0} = 0.15 \)
The yellow pigment has higher solubility in ethanol and weaker interactions with the stationary phase. The second pigment interacts more strongly with the stationary phase (possibly more polar).
Example
Two amino acids, glycine and phenylalanine, are separated using chromatography. The solvent front moves 9.0 cm. Glycine moves 2.7 cm and phenylalanine moves 6.3 cm.
▶️ Answer/Explanation
Glycine:
\( R_f = \dfrac{2.7}{9.0} = 0.30 \)
Phenylalanine:
\( R_f = \dfrac{6.3}{9.0} = 0.70 \)
Glycine is more polar and interacts more with the polar stationary phase (e.g. silica), so it moves less. Phenylalanine is less polar and moves further with the mobile phase.
