How much? The amount of chemical change : R2.1.4 Percentage yield IB DP Chemistry Study Notes - New Syllabus 2025
How much? The amount of chemical change – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 2.1.4 – Percentage Yield
Reactivity 2.1.4 – Percentage Yield
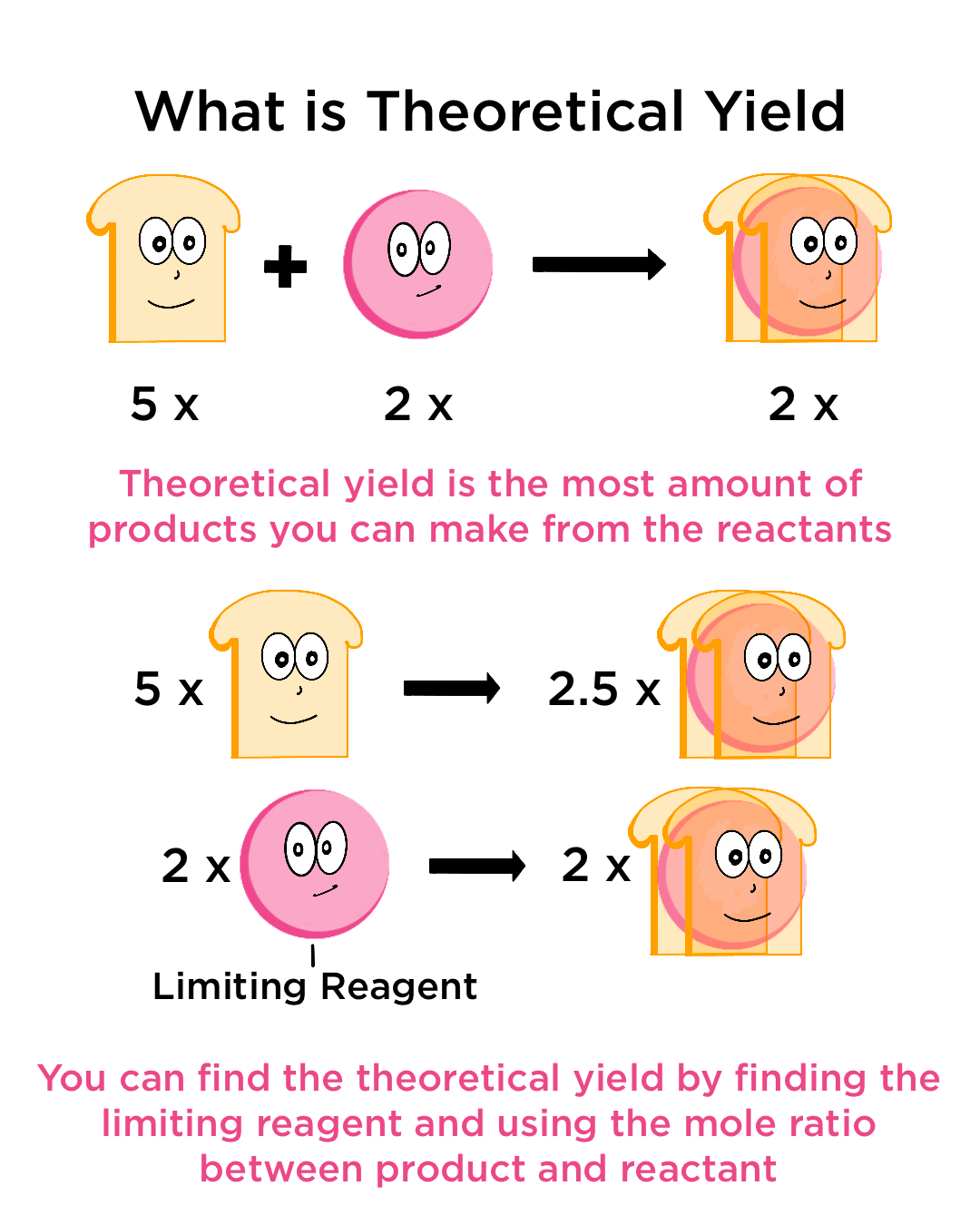
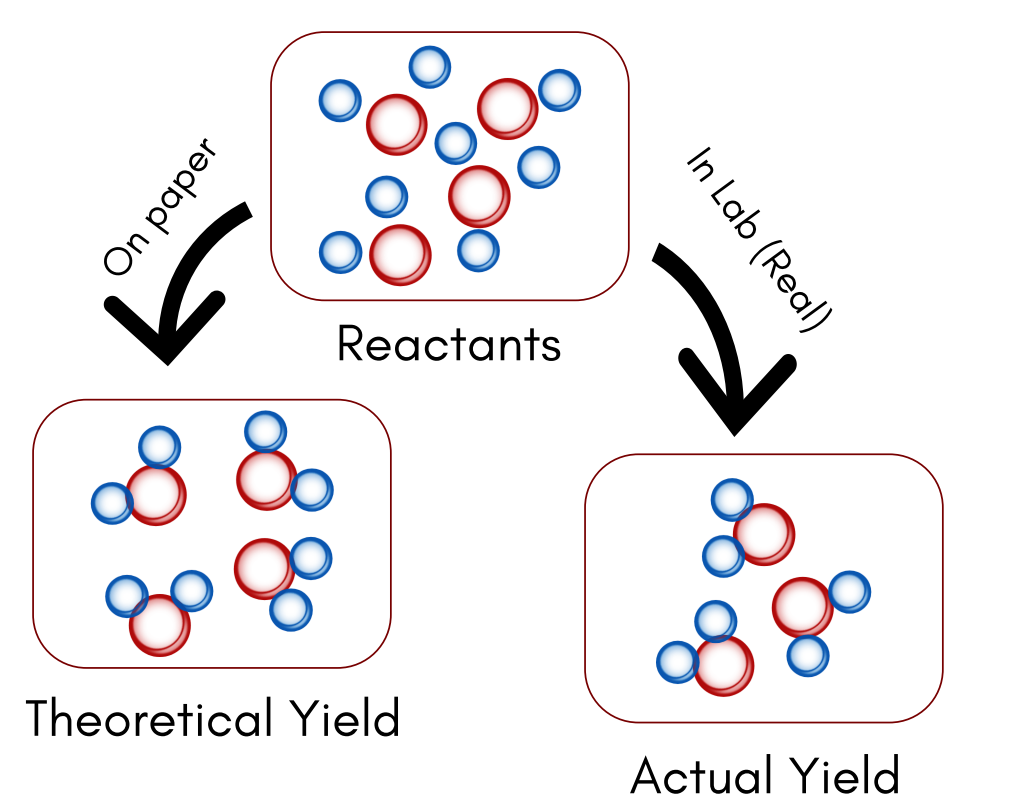
Theoretical Yield
The theoretical yield is the maximum mass of product that can be formed in a chemical reaction based on the amount of limiting reactant. It is calculated using stoichiometry and assumes that the reaction proceeds with 100% efficiency.
Experimental Yield
The experimental yield (also called actual yield) is the amount of product actually obtained from a chemical reaction, as measured in a laboratory or industrial process. This is usually less than the theoretical yield due to losses, incomplete reactions, or side reactions.
Percentage Yield
The percentage yield expresses how efficient a reaction was in producing the product. It is calculated by comparing the actual yield to the theoretical yield:
\( \text{Percentage Yield} = \left( \frac{\text{Experimental Yield}}{\text{Theoretical Yield}} \right) \times 100 \)
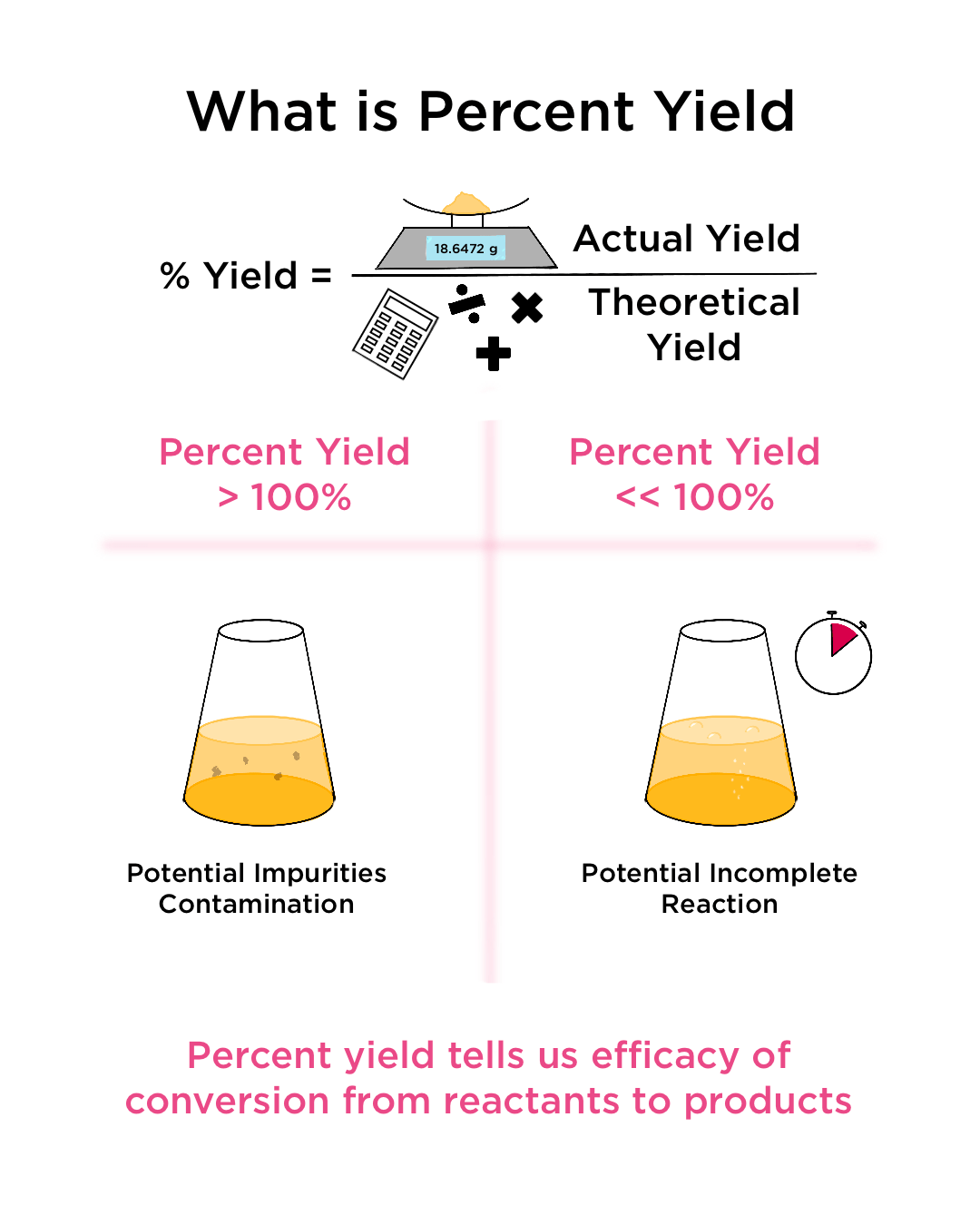
Why Actual Yield Is Often Less Than Theoretical Yield
- Some product may be lost during filtration, evaporation, or transfer
- The reaction may not go to completion
- Side reactions may occur
- Purification steps may reduce yield
Example
In the reaction:
\( \text{Zn} + \text{H}_2\text{SO}_4 \rightarrow \text{ZnSO}_4 + \text{H}_2 \)
If the theoretical yield of hydrogen gas is 0.695 g but the actual yield collected is 0.625 g, calculate the percentage yield.
▶️Answer/Explanation
- \( \text{Percentage Yield} = \left( \frac{0.625}{0.695} \right) \times 100 \approx 89.9\% \)
Key Points
- Theoretical yield is calculated from stoichiometry and limiting reactant.
- \( \text{Percentage Yield} > 100\% \) is usually due to measurement error or impurities.
General Strategy for Solving Reacting Quantity Problems
- Write the balanced chemical equation
- Convert all known masses into moles using \( n = \frac{m}{M} \)
- Determine the limiting reactant by comparing mole ratios
- Use the mole ratio to find moles of product formed
- Calculate the theoretical yield using \( m = n \times M \)
- Use actual (experimental) yield if given, and apply:
\( \text{Percentage Yield} = \left( \frac{\text{Experimental Yield}}{\text{Theoretical Yield}} \right) \times 100 \)
Example
Consider the reaction:
\( 2\text{Na} + \text{Cl}_2 \rightarrow 2\text{NaCl} \)
Given: 4.60 g of Na reacts with 3.20 g of \( \text{Cl}_2 \). The experimental yield of NaCl is 6.00 g. Calculate:
- (a) Limiting reactant
- (b) Theoretical yield of NaCl
- (c) Percentage yield
▶️Answer/Explanation
- Step 1: Molar Masses
- \( M(\text{Na}) = 22.99 \) g/mol → \( n(\text{Na}) = \frac{4.60}{22.99} \approx 0.200 \) mol
- \( M(\text{Cl}_2) = 70.90 \) g/mol → \( n(\text{Cl}_2) = \frac{3.20}{70.90} \approx 0.0451 \) mol
- Step 2: Determine Limiting Reactant
- Equation ratio: \( \text{Na} : \text{Cl}_2 = 2 : 1 \), so 0.200 mol Na requires \( 0.100 \) mol \( \text{Cl}_2 \), but only 0.0451 mol is available → \( \text{Cl}_2 \) is the limiting reactant
- Step 3: Calculate Theoretical Yield
- From the ratio \( \text{Cl}_2 : \text{NaCl} = 1 : 2 \), so \( n(\text{NaCl}) = 2 \times 0.0451 = 0.0902 \) mol
- \( M(\text{NaCl}) = 22.99 + 35.45 = 58.44 \) g/mol
- \( m(\text{NaCl}) = 0.0902 \times 58.44 \approx 5.27 \) g
- Step 4: Calculate Percentage Yield
- Given experimental yield = 6.00 g
- \( \text{Percentage Yield} = \left( \frac{6.00}{5.27} \right) \times 100 \approx 113.8\% \)
- Note: Yield over 100% indicates experimental error or impurities.
Key Tips
- Always start with a balanced chemical equation
- Use \( n = \frac{m}{M} \) and mole ratios to compare reactants
- Identify the limiting reactant to control theoretical yield
- Use experimental data to evaluate reaction efficiency
- Check units and significant figures consistently
