How much? The amount of chemical change : R2.1.5 Atom economy IB DP Chemistry Study Notes - New Syllabus 2025
How much? The amount of chemical change – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 2.1.5 – Atom Economy in Green Chemistry
Reactivity 2.1.5 – Atom Economy in Green Chemistry
Atom Economy
Atom economy is a theoretical measure of how efficiently the atoms in the reactants are used to form the desired product in a chemical reaction. It is expressed as a percentage and considers how much of the total mass of reactants ends up in the useful product(s).
It reflects the principles of green chemistry, which aims to reduce chemical waste and improve the sustainability of chemical processes.
Formula:
\( \text{Atom Economy} = \left( \frac{\text{Molar Mass of Desired Product}}{\text{Total Molar Mass of All Products}} \right) \times 100 \)
Steps for Calculation:
- Write a balanced chemical equation.
- Identify the desired product.
- Calculate the molar mass of the desired product.
- Calculate the total molar mass of all products.
- Use the formula to calculate atom economy.
Purpose and Importance
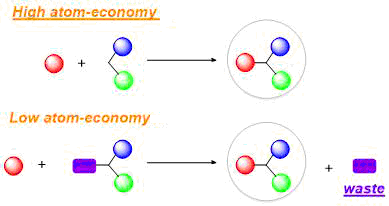
- High atom economy means most of the reactant atoms are incorporated into the desired product.
- Low atom economy indicates more waste and by-products, increasing cost and environmental impact.
- Improving atom economy is essential in industrial chemistry to develop more efficient and sustainable processes.
Atom Economy vs Percentage Yield
- Atom economy is a theoretical measure based on the balanced chemical equation.
- Percentage yield is based on the actual amount of product obtained from a reaction.
- Both are used together to evaluate the effectiveness of a reaction.
Inverse Relationship Between Atom Economy and Waste
A reaction with low atom economy produces more unwanted by-products, leading to greater waste and cost. Conversely, a reaction with high atom economy minimizes waste and is more environmentally friendly.
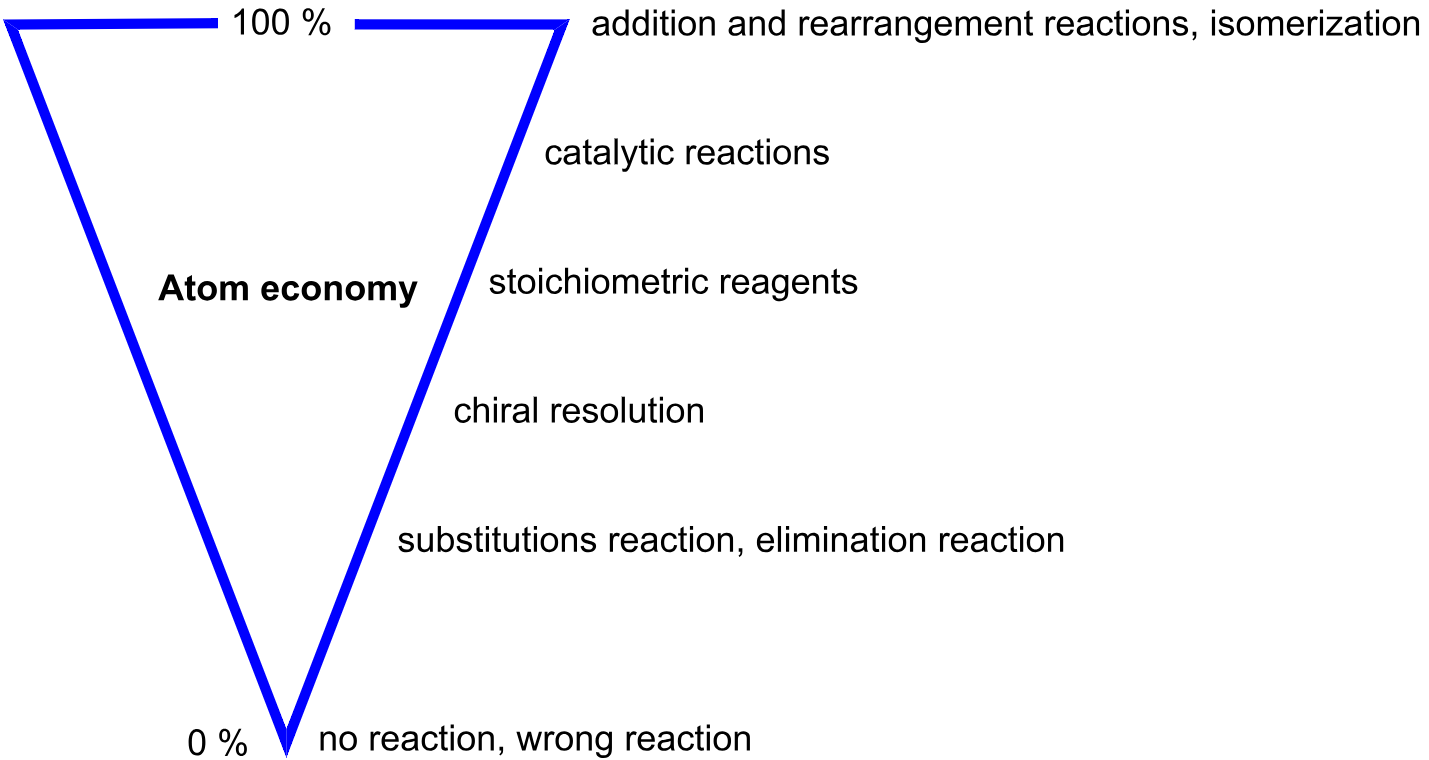
Examples of High Atom Economy:
- Addition reactions: All atoms in the reactants are incorporated into a single product.
- Synthesis of ammonia: \( \text{N}_2 + 3\text{H}_2 \rightarrow 2\text{NH}_3 \)
Examples of Low Atom Economy:
- Substitution and elimination reactions: Often produce multiple products, including waste.
- Example: Preparation of aspirin also produces acetic acid as a by-product.
Example
In the reaction:
\( \text{C}_6\text{H}_5\text{COOH} + (CH_3\text{CO})_2\text{O} \rightarrow \text{C}_9\text{H}_8\text{O}_4 + \text{CH}_3\text{COOH} \)
This is the synthesis of aspirin (desired product: \( \text{C}_9\text{H}_8\text{O}_4 \)) and a by-product acetic acid.
▶️Answer/Explanation
- Molar mass of aspirin = 180.16 g/mol
- Total molar mass of all products = 180.16 + 60.05 = 240.21 g/mol
- Atom economy = \( \left( \frac{180.16}{240.21} \right) \times 100 \approx 74.96\% \)
- This indicates that ~25% of atoms go to waste.
Example
High Atom Economy – Synthesis of Water
\( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \)
▶️Answer/Explanation
- Desired product: \( \text{H}_2\text{O} \)
- Molar mass of \( 2\text{H}_2\text{O} = 2 \times 18.02 = 36.04 \) g/mol
- Total molar mass of products = 36.04 g/mol (only one product)
- Atom Economy = \( \left( \frac{36.04}{36.04} \right) \times 100 = 100\% \)
- This is a reaction with no waste and excellent atom economy.
Example
Low Atom Economy – Production of Ethene from Ethanol
\( \text{C}_2\text{H}_5\text{OH} \rightarrow \text{C}_2\text{H}_4 + \text{H}_2\text{O} \)
▶️Answer/Explanation
- Desired product: Ethene (\( \text{C}_2\text{H}_4 \))
- Molar mass of \( \text{C}_2\text{H}_4 = 28.05 \) g/mol
- Total molar mass of all products = 28.05 (ethene) + 18.02 (water) = 46.07 g/mol
- Atom Economy = \( \left( \frac{28.05}{46.07} \right) \times 100 \approx 60.9\% \)
- This means ~39% of atoms are lost as by-product (water).
Important Notes:
- Atom economy is always calculated from the balanced chemical equation.
- It is independent of the actual experimental yield.
- Only the molar masses of products are used in the denominator.
Improving Atom Economy
- Design reactions that minimize by-products.
- Use catalytic and addition reactions.
- Select synthetic routes that maximize use of all atoms.
