Reactivity How fast? The rate of chemical change : R2.2.1 Rate of reaction IB DP Chemistry Study Notes - New Syllabus 2025
Reactivity How fast? The rate of chemical change – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 2.2.1 – Rate of Reaction
Reactivity 2.2.1 – Rate of Reaction
The rate of a chemical reaction is defined as the change in the concentration of a reactant or product per unit time. It describes how quickly reactants are converted into products in a chemical reaction.
Rate Formula:
\( \text{Rate} = \frac{\Delta[\text{X}]}{\Delta t} \)
- \( \Delta[\text{X}] \) = change in concentration of reactant or product X (mol/dm3)
- \( \Delta t \) = time interval (seconds or minutes)
The units of rate are typically: mol dm−3 s−1
Key Concepts:
- A positive rate refers to the formation of products.
- A negative rate refers to the disappearance of reactants (since concentration decreases).
- The rate of reaction usually changes over time — it is fastest at the start and slows as reactants are used up.
Example
Consider the reaction: \( \text{Mg}(s) + 2\text{HCl}(aq) \rightarrow \text{MgCl}_2(aq) + \text{H}_2(g) \)
If the concentration of HCl decreases from 1.00 mol/dm3 to 0.60 mol/dm3 in 40 seconds, what is the average rate of reaction?
▶️Answer/Explanation
- \( \Delta[\text{HCl}] = 0.60 – 1.00 = -0.40 \) mol/dm3 (negative because it’s a reactant)
- \( \Delta t = 40 \) s
- \( \text{Rate} = \frac{-0.40}{40} = -0.010 \) mol dm−3 s−1
- Magnitude of rate = 0.010 mol dm−3 s−1
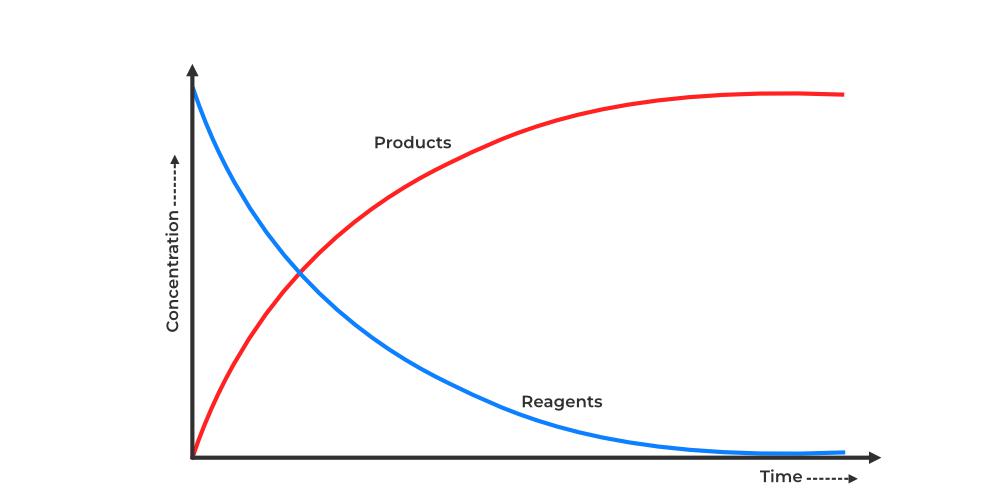
Graphical Representation
- Rate can be shown as the slope of a concentration vs. time graph.
- The steeper the slope, the faster the reaction.
- As the graph flattens, the reaction is slowing down.
Instantaneous vs Average Rate
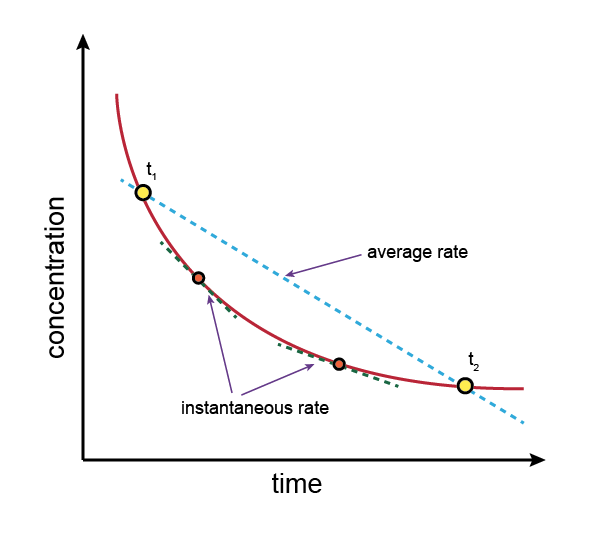
- Average rate: change in concentration over a longer time period
- Instantaneous rate: rate at a specific point in time (slope of tangent to the curve)
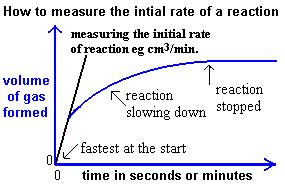
Calculating Reaction Rates from Graphs
Reaction rates can be determined from experimental data plotted on graphs, such as concentration, mass, or volume versus time. The rate at any point is the gradient (slope) of the curve at that point. For non-linear curves, this involves drawing a tangent.
Types of Graphs Used:
- Concentration vs. time – used for solutions (mol/dm3)
- Volume vs. time – used when a gas is produced (cm3 or dm3)
- Mass vs. time – used when mass of reactants/products changes (grams)
General Formula for Rate:
\( \text{Rate} = \frac{\Delta y}{\Delta t} = \text{slope of the graph} \)
- \( y \) = measured quantity (e.g. concentration, mass, volume)
- \( t \) = time (usually in seconds)
1. Concentration vs Time
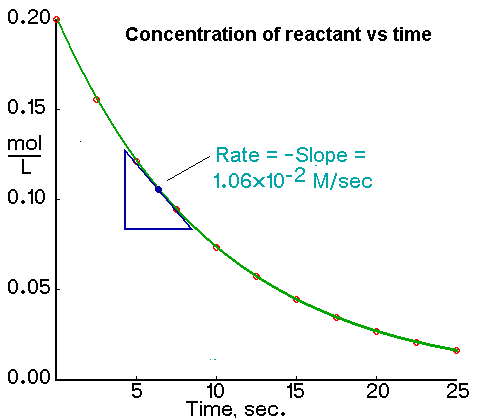
- The slope gives the rate of change of concentration.
- Use this for reactions in solution.
Example
If a concentration–time graph for a reaction shows a drop from 1.00 mol/dm3 to 0.70 mol/dm3 over 30 seconds, the average rate is:
▶️Answer/Explanation
\( \Delta[\text{Reactant}] = 0.70 – 1.00 = -0.30 \) mol/dm3
\( \Delta t = 30 \) s
\( \text{Rate} = \frac{-0.30}{30} = -0.010 \) mol dm−3 s−1
(The magnitude of the rate is 0.010 mol dm−3 s−1)
2. Volume vs Time
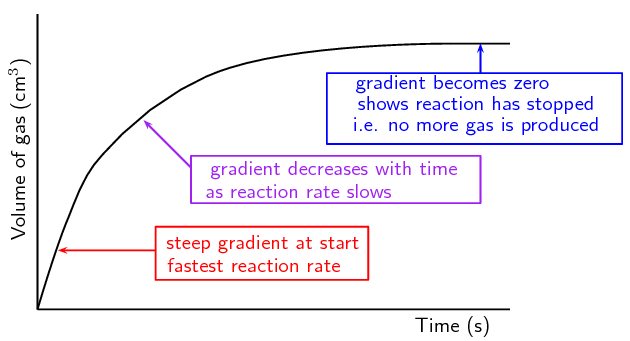
- Used when a gas is produced and collected (e.g. hydrogen in a reaction).
- The slope of the tangent to the curve gives the rate of gas production.
Example
A reaction produces 60.0 cm3 of gas in the first 20 seconds. What is the rate of reaction?
▶️Answer/Explanation
\( \Delta V = 60.0 \) cm3, \( \Delta t = 20 \) s
Rate = \( \frac{60.0}{20} = 3.0 \) cm3/s
3. Mass vs Time
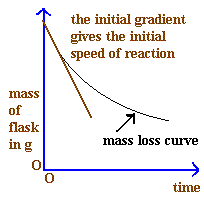
- Used when the mass of a reactant/product changes during the reaction (e.g. gas escapes and mass decreases).
- Rate is calculated from how fast mass decreases with time.
Example
In a decomposition reaction, mass drops from 5.00 g to 3.75 g in 15 seconds.
▶️Answer/Explanation
\( \Delta m = 5.00 – 3.75 = 1.25 \) g
\( \Delta t = 15 \) s
Rate = \( \frac{1.25}{15} \approx 0.083 \) g/s
4. Using a Tangent to Find Instantaneous Rate
If the graph is a curve, the rate at a specific time is found by drawing a tangent to the curve at that point:
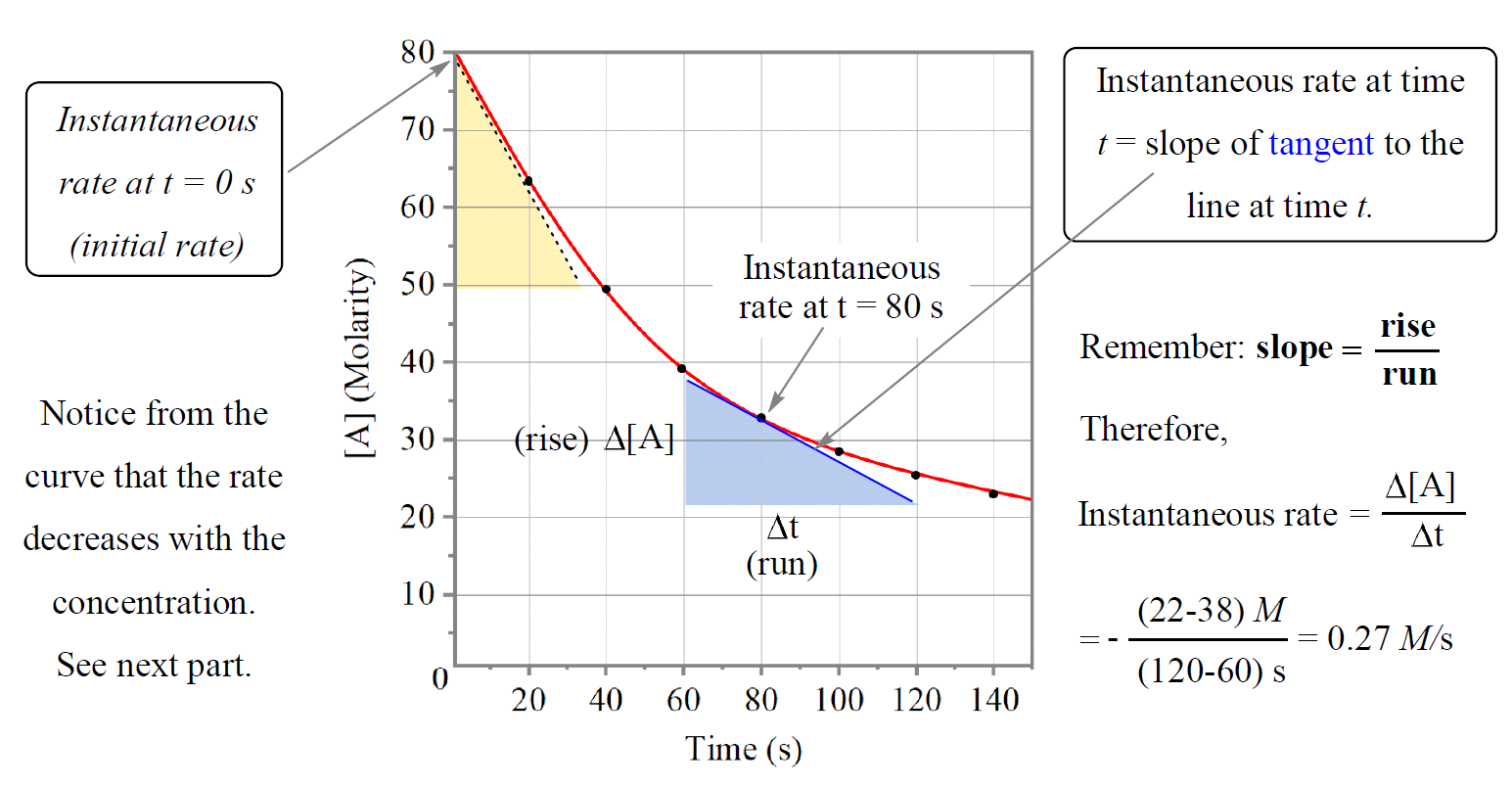
- Draw a straight line that just touches the curve at the desired point without cutting it.
- Choose two points on the tangent to calculate the rise and run.
- Use the formula: \( \text{Rate} = \frac{\text{rise}}{\text{run}} \)
Measuring Rates of Reaction
Why Concentration is Not Usually Measured Directly
In most school or lab experiments, it is difficult to directly measure the concentration of reactants or products at every instant. Instead, indirect measurements are used, which relate to changes in mass, volume, or physical properties over time.
Common Experimental Methods Used to Determine Reaction Rates
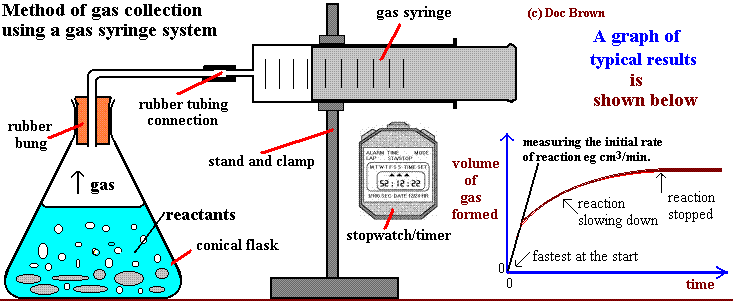
- Measuring Volume of Gas Evolved (Gas Syringe or Water Displacement)
Used for reactions that produce a gas (e.g. reaction of metals with acids)
Volume is recorded at regular time intervals - Measuring Mass Loss (on a Balance)
Used when gas escapes from an open container
Total mass of reaction mixture decreases over time - Colorimetry
Measures the change in color intensity of a solution
Useful in reactions involving colored substances (e.g. transition metal ions) - pH Measurement
Used for acid-base reactions
pH is monitored using a probe or indicator to estimate concentration of H+ ions - Conductivity Measurements
Electrical conductivity changes as ionic concentration changes
Effective for reactions in aqueous solution involving ions - Precipitate Formation – Disappearing Cross Method
Used when a precipitate forms (e.g. \( \text{Na}_2\text{S}_2\text{O}_3 + \text{HCl} \))
Time taken for a mark (e.g. black cross) beneath the reaction flask to disappear is measured
Time as a Variable in Rate Experiments
i) Time as a Dependent Variable
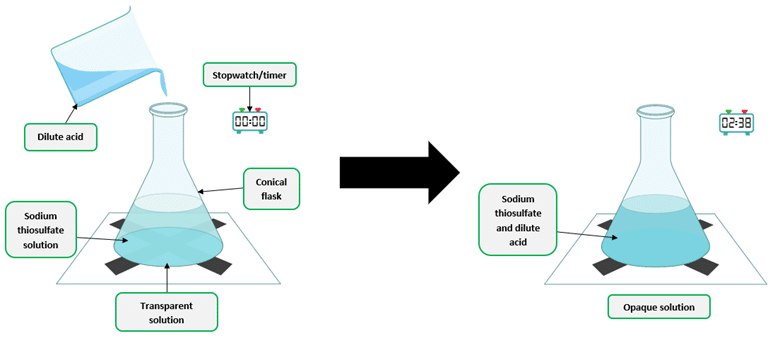
- Time is measured as a result of the reaction’s progress.
- Example: Measuring how long it takes for a visible change to occur (e.g. color change, formation of a precipitate).
- Common in: “Disappearing cross” method or clock reactions.
ii) Time as an Independent Variable
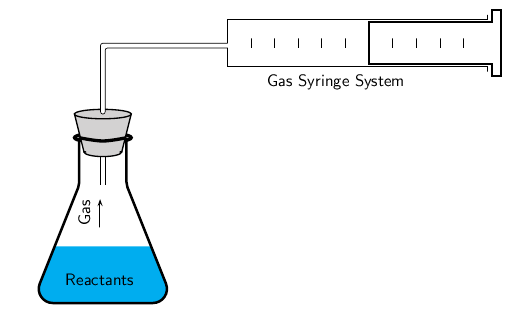
- Time is controlled and used to measure changes in properties at intervals.
- Example: Measuring gas volume or mass lost at fixed time intervals.
- Common in: gas syringe experiments, loss of mass over time, or spectrophotometry.
