Reactivity How fast? The rate of chemical change : R2.2.7 Energy profiles and transition states IB DP Chemistry Study Notes - New Syllabus 2025
Reactivity How fast? The rate of chemical change – IB DP Chemistry- Study Notes
IITian Academy excellent Introduction to the Particulate Nature of Matter – Study Notes and effective strategies will help you prepare for your IB DP Chemistry 2025 exam.
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Chemistry 2025 HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
Reactivity 2.2.7 – Energy Profiles and the Rate-Determining Step
Reactivity 2.2.7 – Energy Profiles and the Rate-Determining Step
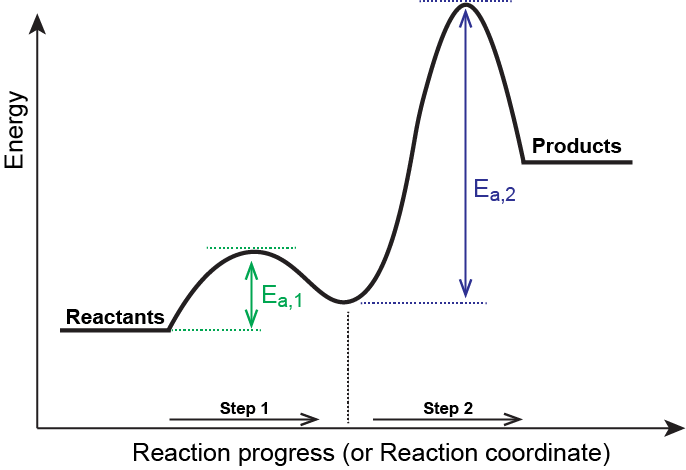
Definition – Energy Profile
An energy profile (or reaction coordinate diagram) is a graphical representation of the energy changes that occur during the progress of a chemical reaction. It shows how the potential energy of the system varies with the reaction coordinate (progress of reaction from reactants to products).
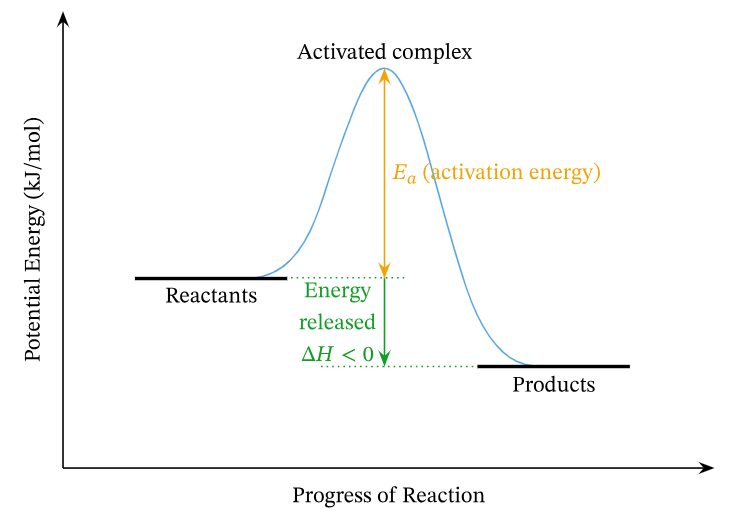
Key Features of an Energy Profile
- Reactants and Products: Represented on the y-axis (potential energy).
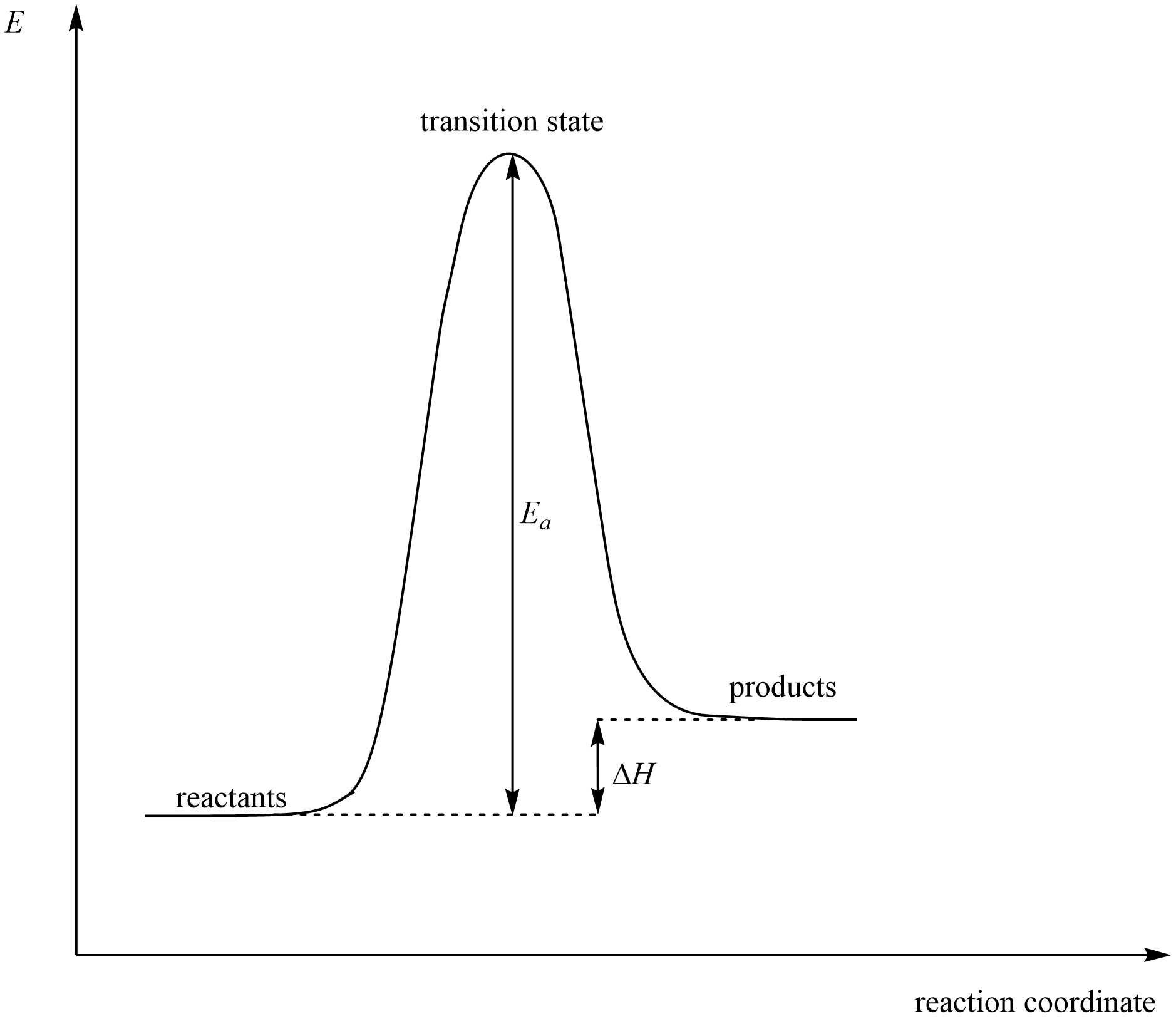
- Activation Energy (Ea): The energy barrier that must be overcome for the reaction to proceed. It is the difference in energy between the reactants and the transition state.
- Transition State: A high-energy, unstable arrangement of atoms at the top of the energy barrier. This is where bonds are partially broken and formed. It is denoted by a peak on the diagram.
- Enthalpy Change (ΔH): The difference in energy between the reactants and products. It indicates whether the reaction is endothermic (ΔH > 0) or exothermic (ΔH < 0).
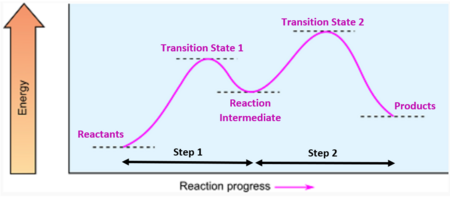
Single-Step vs. Multistep Reactions
- Single-Step Reaction: Has one activation energy and one transition state. The entire reaction occurs in a single elementary step.
- Multistep Reaction: Involves two or more elementary steps, each with its own activation energy and transition state. These reactions show multiple peaks and valleys on the energy profile.
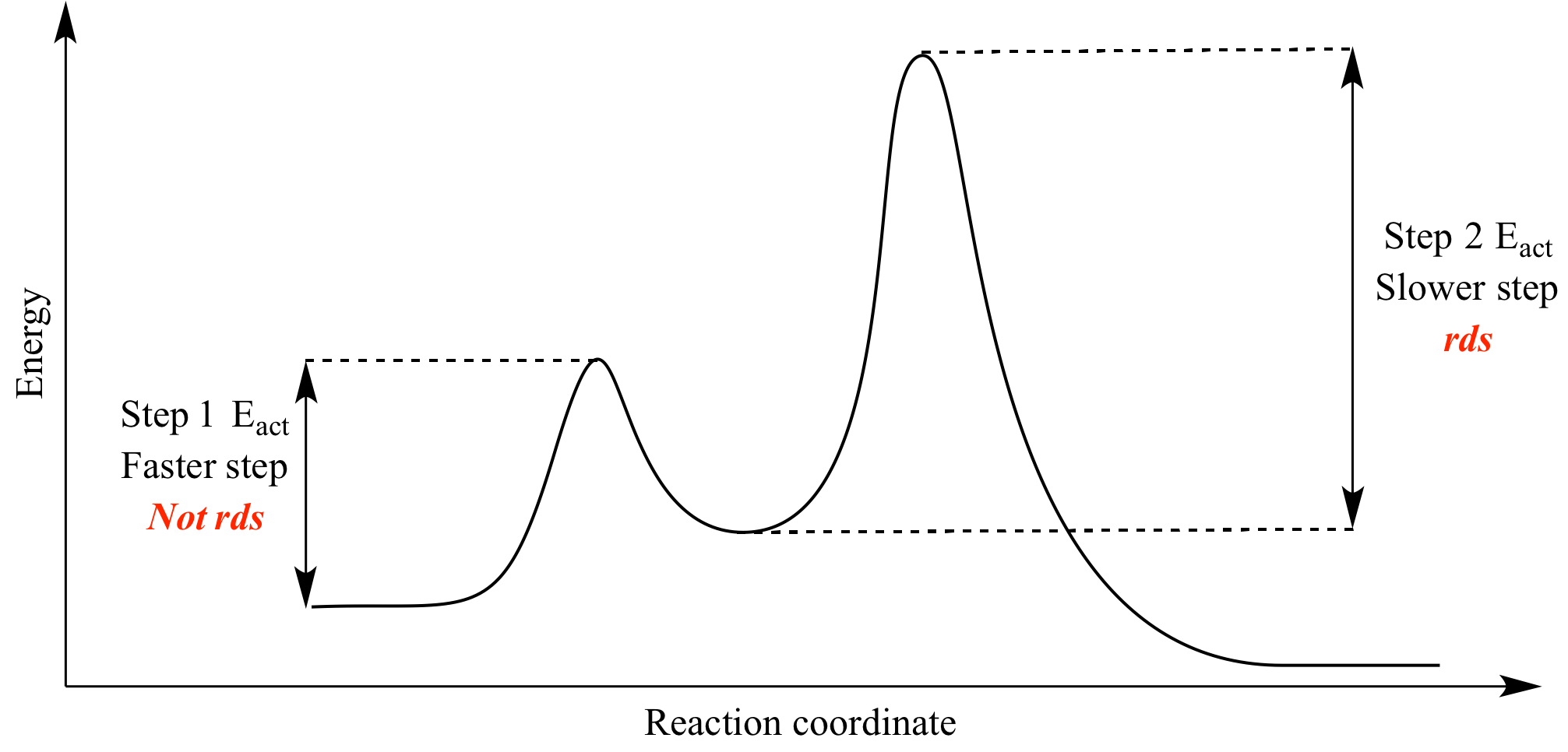
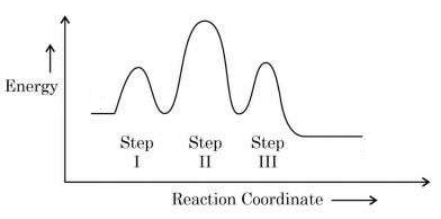
Rate-Determining Step (RDS)
- The rate-determining step is the slowest step in a multistep reaction mechanism.
- It has the highest activation energy of all steps.
- This step controls the overall rate of the reaction.
- On an energy profile, the rate-determining step corresponds to the tallest energy peak.

Identifying the Rate-Determining Step in Energy Profiles
- Each step in a multistep reaction corresponds to a rise and fall in energy (peak = transition state, valley = intermediate).
- Compare the activation energies (heights of the peaks).
- The highest peak indicates the step requiring the most energy to proceed → the slowest → the RDS.
Construct and Interpret Energy Profiles from Kinetic Data
1. Connecting Kinetics to Energy Profiles
- The rate law reveals which reactants are involved in the rate-determining step (RDS).
- The number of steps in the reaction mechanism corresponds to the number of peaks in the energy profile.
- Each peak corresponds to a transition state; each valley between them represents a reaction intermediate.
- The highest peak corresponds to the step with the highest activation energy → this is the RDS.

Transition States in Multistep Reactions
- Every step in a multistep reaction has its own transition state.
- Transition states are extremely short-lived and cannot be isolated.
- They represent the point of maximum energy for a given step.
2. Interpreting Features of Energy Profiles
| Feature | What It Represents |
|---|---|
| Peak (Transition State) | Unstable, high-energy arrangement of atoms during bond breaking/forming |
| Valley (Intermediate) | Species formed in one step and consumed in the next; more stable than TS |
| Height of Peak | Activation energy for that step |
| Reactant-to-Product Difference | Enthalpy change \( (\Delta H) \) |
| Highest Peak | Rate-determining step (RDS) |
3. How to Construct an Energy Profile from Kinetic Data
- Step 1: Determine the number of steps from the proposed mechanism.
- Step 2: Identify the rate-determining step from the rate law (the step involving the species in the rate equation).
- Step 3: Assign each step a relative activation energy (taller peak for slower step).
- Step 4: Mark transition states (peaks) and intermediates (valleys).
- Step 5: Ensure that the overall enthalpy change (ΔH) matches experimental observations (exothermic or endothermic).
4. Key Relationships Between Graphs and Mechanisms
- Rate law ≠ overall equation: It only reflects the RDS.
- Presence of intermediates: Indicated by valleys between peaks.
- More steps → more peaks: Each elementary step has a transition state.
Example:
A reaction takes place in three steps:
- \( \text{A + B} \rightarrow \text{C} \) (fast)
- \( \text{C} \rightarrow \text{D} \) (slow)
- \( \text{D + E} \rightarrow \text{F} \) (fast)
Kinetic data shows that the rate law is \( \text{Rate} = k[\text{C}] \). You are shown an energy profile with three peaks. What information about the mechanism, intermediates, and the rate-determining step can be deduced from the profile and the kinetic data?
▶️Answer/Explanation
- The mechanism has three steps → the energy profile should show three transition states (three peaks) and two intermediates (valleys).
- The rate law only involves [C], meaning Step 2 is the rate-determining step and should have the highest peak (activation energy).
- Species C and D are intermediates—formed and consumed within the mechanism—and appear in the valleys between peaks.
- Step 2 controls the overall rate; therefore, the energy barrier for this step is largest.
Example:
A reaction follows the overall equation: \( \text{2NO}_2 + \text{F}_2 \rightarrow 2\text{NO}_2\text{F} \) The reaction mechanism is proposed as follows:
- Step 1 (fast equilibrium): \( \text{NO}_2 + \text{F}_2 \rightleftharpoons \text{NO}_2\text{F} + \text{F} \)
- Step 2 (slow): \( \text{NO}_2 + \text{F} \rightarrow \text{NO}_2\text{F} \)
Answer the following:
- How many elementary steps does this reaction involve?
- Identify the intermediate and explain how you can recognize it.
- Which is the rate-determining step, and how can you tell?
- Explain how the activation energy would differ between Step 1 and Step 2 based on their relative speeds.
- Describe how this mechanism is consistent with the observed rate law: \( \text{Rate} = k[\text{NO}_2][\text{F}_2] \)
▶️Answer/Explanation
- Steps: Two elementary steps (Step 1 and Step 2).
- Intermediate: The fluorine atom (\( \text{F} \)) is produced in Step 1 and consumed in Step 2. Intermediates appear in the mechanism but not in the overall reaction.
- Rate-determining step: Step 2 is slow, so it determines the overall rate.
- Activation energy: Step 2 has higher activation energy than Step 1, as higher activation energy usually corresponds to a slower step.
- Rate law consistency: Since Step 1 is fast and reversible and Step 2 is slow, the rate depends on the concentrations of \( \text{NO}_2 \) and \( \text{F}_2 \), consistent with the observed rate law.
Example:
The following mechanism is proposed for the reaction between hydrogen peroxide and iodide ions:
- Step 1: \( \text{H}_2\text{O}_2 + \text{I}^- \rightarrow \text{IO}^- + \text{H}_2\text{O} \)
- Step 2: \( \text{IO}^- + \text{H}_2\text{O}_2 \rightarrow \text{I}^- + \text{O}_2 + \text{H}_2\text{O} \)
Fill in the table below based on your understanding of the mechanism:
| Species | Appears in Overall Equation? | Role | Explanation |
|---|---|---|---|
| \( \text{H}_2\text{O}_2 \) | Yes | Reactant | Used in both steps; consumed overall |
| \( \text{I}^- \) | No | Catalyst | Consumed in Step 1, regenerated in Step 2 |
| \( \text{IO}^- \) | No | Intermediate | Formed in Step 1, used up in Step 2 |
| \( \text{O}_2 \) | Yes | Product | Formed in Step 2; appears in overall reaction |
▶️Answer/Explanation
- Catalyst: \( \text{I}^- \) – used and regenerated.
- Intermediate: \( \text{IO}^- \) – appears in mechanism but not in overall equation.
- Reactants and products: Identified based on overall net consumption/formation across steps.