Question
A jar of air was placed upside down on top of a jar containing a brown gas as shown.
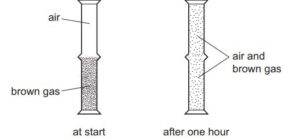
Which process has taken place?
diffusion both upwards and downwards
diffusion downwards only
diffusion upwards only
diffusion and osmosis
▶️Answer/Explanation
A
The correct process that has taken place in this scenario is option A: diffusion both upwards and downwards.
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. In this case, the jar containing the brown gas has a higher concentration of gas particles compared to the surrounding air. When the jar of air is placed upside down on top of the jar containing the gas, diffusion occurs in both directions.
Initially, the concentration of the brown gas is higher inside the jar compared to the outside air. As a result, gas particles from the jar will diffuse outwards, spreading into the surrounding air. At the same time, the air from the surrounding environment, which has a lower concentration of gas particles, will diffuse into the jar, moving inwards.
Therefore, both upward and downward diffusion of gas particles occur in this situation.
Question
A frog is an animal. A frog’s skin is permeable to oxygen and carbon dioxide.
When a frog is swimming in pond water, in which directions will there be a net diffusion of oxygen
and carbon dioxide?
▶️Answer/Explanation
A
When a frog is swimming in pond water, there will be a net diffusion of oxygen into the frog’s body and a net diffusion of carbon dioxide out of the frog’s body.Frogs have a unique respiratory system that allows them to obtain oxygen through their skin. When a frog is in the water, oxygen dissolved in the water can pass through the frog’s moist skin and enter its bloodstream. This process is known as cutaneous respiration.
The net diffusion of oxygen occurs from the pond water into the frog’s body because there is a higher concentration of dissolved oxygen in the water compared to the oxygen concentration in the frog’s bloodstream. Oxygen moves from an area of higher concentration (pond water) to an area of lower concentration (frog’s body) through the process of diffusion.
Conversely, carbon dioxide produced by the frog’s body cells will diffuse out of the frog’s bloodstream and across its skin into the pond water. Carbon dioxide concentration is higher in the frog’s body compared to the water, so it moves from an area of higher concentration (frog’s body) to an area of lower concentration (pond water) through diffusion.
Therefore, during swimming in pond water, there will be a net diffusion of oxygen into the frog’s body and a net diffusion of carbon dioxide out of the frog’s body.
Question
The diagram shows a test-tube containing clear jelly. A drop of blue ink is injected into the middle
of the jelly.
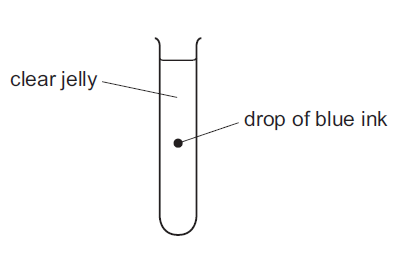
The blue colour of the ink spreads throughout the jelly.
By which process does the blue ink spread through the jelly?
A active transport
B catalysis
C diffusion
D osmosis
▶️Answer/Explanation
C
The process by which the blue ink spreads through the jelly in the test tube is called diffusion. Diffusion is the passive movement of particles or molecules from an area of higher concentration to an area of lower concentration. In this case, the blue ink, which has a higher concentration in the center of the jelly, will naturally diffuse or spread outwards, gradually mixing with the clear jelly until it becomes evenly distributed. Therefore, the correct answer is C: diffusion.
Question
The apparatus shown was set up.
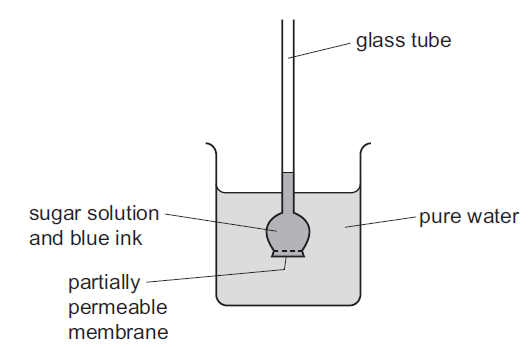
Some hours later, the water in the beaker had turned blue, and the liquid in the glass tube had
moved upwards.
Which processes caused these changes?

▶️Answer/Explanation
D
The processes that caused the changes in the apparatus are according to option D.
1. Water turning blue: Diffusion
Diffusion is the process by which particles or molecules move from an area of higher concentration to an area of lower concentration. In this case, it is likely that a substance with a blue color dissolved in the water in the beaker. Over time, the blue-colored particles diffused through the water, causing it to turn blue uniformly.
2. Liquid in the glass tube moving upwards: Osmosis
Osmosis is the process of water molecules moving across a semi-permeable membrane from an area of lower solute concentration to an area of higher solute concentration. In the apparatus shown, the glass tube is likely filled with a liquid that has a higher solute concentration compared to the water in the beaker. As a result, water molecules from the beaker moved across the semi-permeable membrane of the tube to dilute the higher solute concentration, causing the liquid in the tube to rise.
Question
What is an example of diffusion?
A dust particles being moved by ciliated cells in the trachea
B oxygen molecules moving into a red blood cell in the lungs
C pollen grains moving from anthers to stigmas in the wind
D red blood cells moving in a blood capillary in a muscle
▶️Answer/Explanation
B
An example of diffusion is option B: oxygen molecules moving into a red blood cell in the lungs. Diffusion refers to the movement of molecules or particles from an area of higher concentration to an area of lower concentration, resulting in their even distribution. In this example, oxygen molecules diffuse from an area of higher concentration in the alveoli of the lungs into the red blood cells, where their concentration is lower. This diffusion process allows for the exchange of gases and oxygenation of the blood.
