Question
The diagram represents two liquids separated by a partially permeable membrane.

Which statement describes how the molecules will move by osmosis?
(B) Molecules of the dissolved substance move from right to left.
(C) Overall, water molecules move from left to right.
(D) Overall, water molecules move from right to left.
▶️ Answer/Explanation
✅ Answer: (C)
Question
Some pieces of potato plant tissue are placed in a very concentrated sugar solution. Other pieces of potato plant tissue are placed in distilled water.
What happens to the mass of the potato pieces in the two liquids?

▶️ Answer/Explanation
This question tests the concept of osmosis, which is the net movement of water molecules from a region of higher water potential to a region of lower water potential through a partially permeable membrane.
In the concentrated sugar solution (hypertonic), the water potential outside the potato cells is lower than inside. Consequently, water leaves the potato cells by osmosis, causing the mass to decrease.
In distilled water (hypotonic), the water potential outside is higher than inside the potato cells. Water enters the cells by osmosis, causing the cells to become turgid and the overall mass to increase.
✅ Answer: (B)
Question
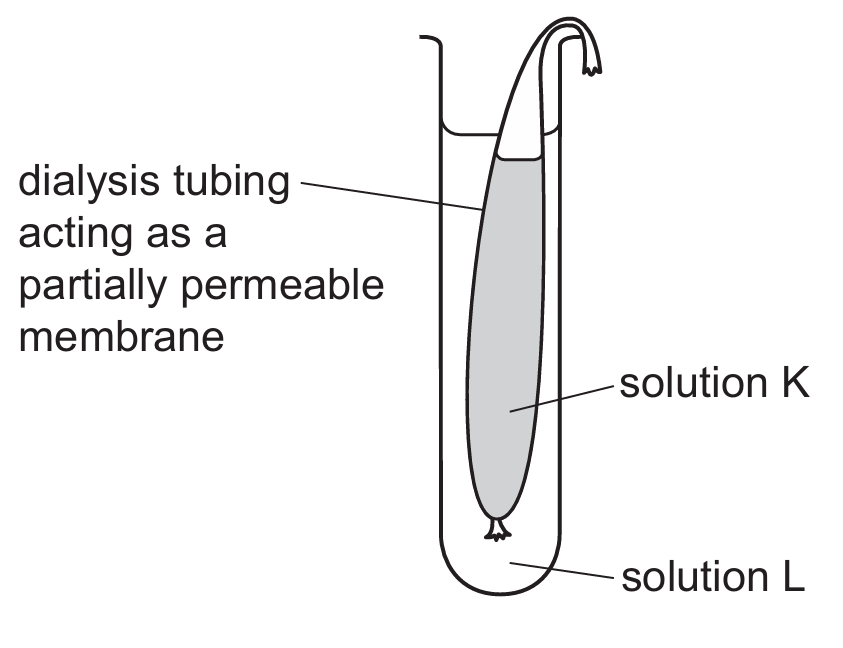
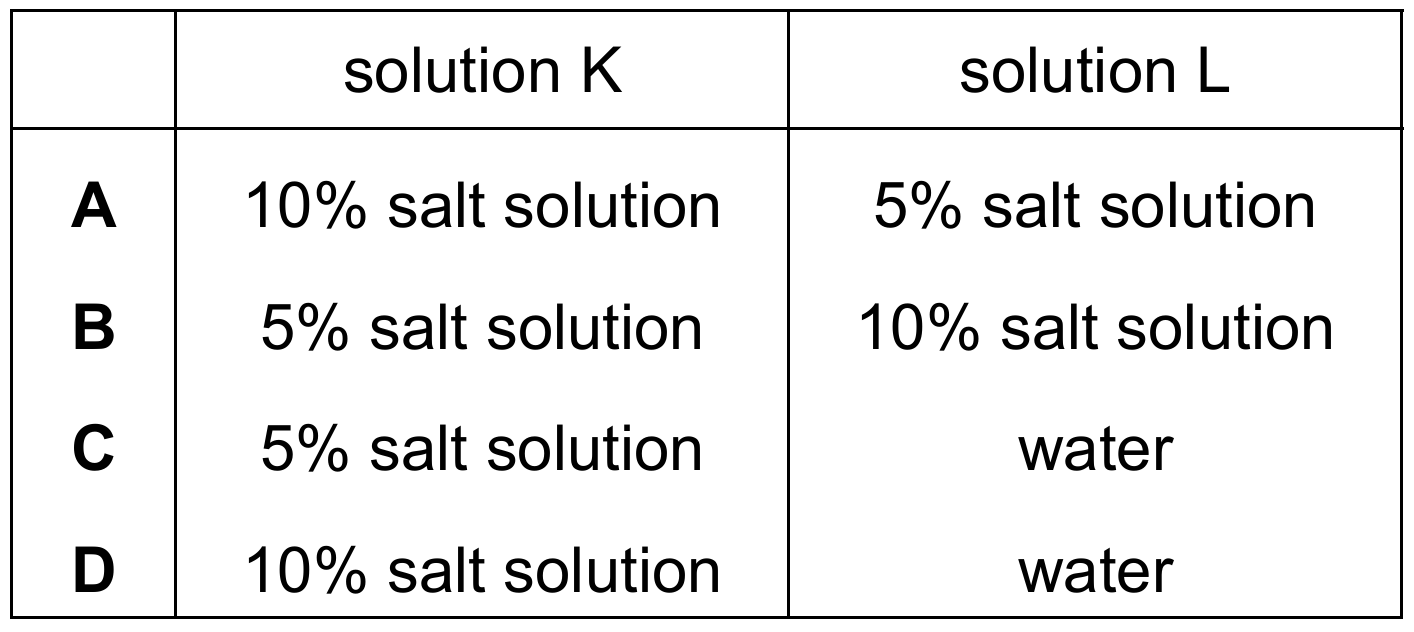
▶️ Answer/Explanation
The correct answer is B.
1. Analyze the Change in Mass:
The mass of the dialysis tubing decreased from \(11.2\text{ g}\) to \(9.4\text{ g}\). A decrease in mass indicates that water has moved out of the dialysis tubing (Solution K) and into the surrounding beaker (Solution L).
2. Apply the Principles of Osmosis:
Osmosis is the net movement of water molecules from a region of higher water potential (dilute solution) to a region of lower water potential (concentrated solution) through a partially permeable membrane.
3. Determine Concentration Gradients:
For water to leave the tubing, the water potential inside the tubing must be higher than the water potential outside. In terms of solute concentration:
- Solution K (Inside): Must have a lower salt concentration (more water).
- Solution L (Outside): Must have a higher salt concentration (less water).
