Which fuel is manufactured by fermentation?
A) diesel
B) ethanol
C) hydrogen
D) kerosene
▶️ Answer/Explanation
Ans: B
Let’s analyze each option:
A) Diesel: Produced from fractional distillation of crude oil, not by fermentation.
B) Ethanol: Can be produced by fermentation of sugars by yeast. This is a well-known biological process where sugars are converted to ethanol and carbon dioxide.
C) Hydrogen: Typically produced through electrolysis of water or from natural gas, not by fermentation.
D) Kerosene: Like diesel, it’s obtained from fractional distillation of crude oil.
Therefore, ethanol is the correct answer as it’s the only fuel listed that’s commonly produced through fermentation.
What are the products formed when glucose is fermented?
A) ethanol and carbon dioxide
B) ethanol and oxygen
C) ethene and carbon dioxide
D) ethene and oxygen
▶️ Answer/Explanation
Ans: A
The fermentation of glucose is represented by the following equation:
C6H12O6 → 2C2H5OH + 2CO2
This shows that glucose breaks down into ethanol (C2H5OH) and carbon dioxide (CO2).
Key points to remember:
- Fermentation is an anaerobic process (doesn’t require oxygen)
- It’s carried out by yeast or certain bacteria
- The bubbles in bread and beer come from the CO2 produced
- Ethene is not a product of fermentation (eliminates options C and D)
Which structure represents a molecule of ethanol?
A) 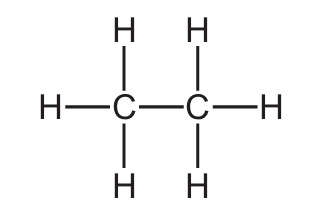
B) 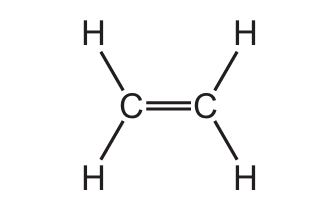
C) 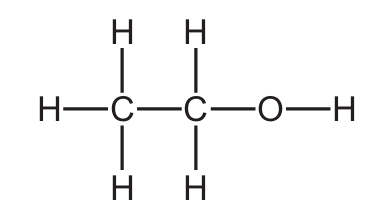
D) 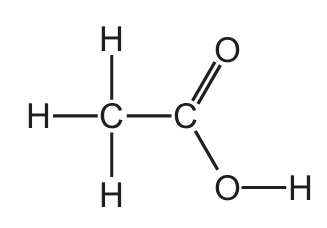
▶️ Answer/Explanation
Ans: C
Ethanol has the molecular formula C2H5OH, which can be represented as CH3-CH2-OH.
Let’s analyze each option:
- A) H-C≡C-H: This is ethyne (acetylene), a triple-bonded hydrocarbon.
- B) H-C=C-H: This is ethene, a double-bonded hydrocarbon.
- C) H-C-C-O-H: This correctly shows ethanol with two carbon atoms, single bonds, and an -OH group.
- D) H-C-O-C-H: This represents an ether (methoxymethane), not ethanol.
The key features of ethanol’s structure are:
- Two carbon atoms with single bonds
- An -OH (hydroxyl) functional group
- No double or triple bonds
Which statement describes how ethanol is manufactured from ethene?
A) Steam is added to ethene using an acid catalyst at 30 °C.
B) Steam is added to ethene using an acid catalyst at 300 °C.
C) Ethene is fermented using yeast at 30 °C.
D) Ethene is fermented using yeast at 300 °C.
▶️ Answer/Explanation
Ans: B
Ethanol can be produced from ethene through hydration, where steam is added to ethene in the presence of an acid catalyst (usually phosphoric acid) at high temperature (around 300°C). This is an industrial method of ethanol production. Options C and D are incorrect because fermentation uses sugars, not ethene. Option A has the right reactants but the temperature is too low – 30°C would be appropriate for fermentation but not for the hydration of ethene.
